The reaction of a compound with silver nitrate in ethanol is used as a chemical test to
Question:
The reaction of a compound with silver nitrate in ethanol is used as a chemical test to determine if the compound is an alkyl halide. The formation of a precipitate of the silver halide constitutes a positive test.
(a) Explain why these conditions favor the SN1 mechanism.
(b) Which of these halides would give a precipitate more rapidly when reacted with AgNO3 in ethanol?
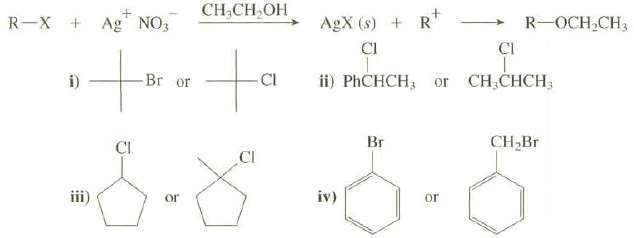
Transcribed Image Text:
AgX (s) + R* CI CH,CH,OH R-X + Ag NO3 R-OCH CH3 CI -Br or CI ii) PHCHCH; or CH,CHCH3 i) Br CH,Br Cl CI iii) or or iv)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (16 reviews)
a The solvent ethanol is polar and there are no strong nucleophiles pr...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Determine the structure of a compound with molecular formula C 5 H 10 O that exhibits the following broadband-decoupled and DEPT-135 spectra. The DEPT-90 spectrum has no signals. Broadband-decoupled...
-
The IR spectrum of a compound with molecular formula C5H8O was obtained in CCl4 and is shown in Figure 13.42. Identify the compound. Wavelenga qum) 15 16 14 3600 340) 3800 3300 3000 280K 2600 2400...
-
A ketone can be prepared from the reaction of a nitrile with a Grignard reagent. Describe the intermediate that is formed in this reaction, and explain how it can be converted to a ketone.
-
Scandinavo Ltd. is a CCPC that began operations on January 1, 2020 when it was first incorporated and a calendar fiscal period was chosen. Scandinavo Ltd. Is not associated with any other...
-
Woodchuck Timber Pty Ltd grows, harvests and processes timber for use in the building industry. The following data relate to the company's sawmill during June: Work in process, 1 June: Direct...
-
Echo, Inc., purchased 10 percent of Pro-Form Corporation on January 1, 2017, for $345,000 and accounted for the investment using the fair-value method. Echo acquires an additional 15 percent of...
-
Which of the four methods for handling missing data would tend to lead to an underestimate of the spread (e.g., SD) of the variable? What are some benefits to this method?
-
The Long-Term Debt section of Rodman Companys balance sheet as of December 31, 2010, included 8% bonds payable of $300,000 less unamortized discount of $22,000. Further examination revealed that...
-
How should fund information be reported in governmental and enterprise fund financial statements? A) Only the major funds should be reported, with separate columns for each fund B) All funds should...
-
what are the features detected by modernizr? CSS Reflections i. ii. iii. Web Workers SNIL iv. IndexedDB a. i and ii b. i, ii and iii c. i, ii and iv d. ii, iii and iv
-
Ethers can be cleaved by treatment with strong acids. Show all of the steps in the mechanism for this reaction and explain why these products are formed rather than iodomethane and2-methyl-2-butanol:...
-
The reaction of an alkyl chloride (or bromide) with sodium iodide in acetone proceeds according to the following equation: Sodium iodide is soluble in acetone, whereas both sodium chloride and sodium...
-
Define internal rate of return. When is it appropriate to use IRR rather than the HPR to measure the return on an investment?
-
The air in an automobile tire with a volume of \(0.015 \mathrm{~m}^{3}\) is at \(30^{\circ} \mathrm{C}\) and \(140 \mathrm{kPa}\) (gage). Determine the amount of air that must be added to raise the...
-
Convex Productions has just received a contract to film a commercial video that will air during a major sporting event in North America, and then be available on-demand through banner advertisements...
-
The following data (and annotations) for March 2016 are for the work in process account of the first of Olympus Companys four departments used in manufacturing its nly product. Assuming that Olympus...
-
If relative volatility can be assumed constant over the change in concentration for each fraction, Eq. \((9-13)\) can be adapted to the collection of fractions from a simple binary batch...
-
(a) Design a PI controller for Problem 8.6-4(b). (b) Design a PD controller for Problem 8.6-4(c). (c) Use the results of parts (a) and (b) to repeat Problem 8.6-4(d). Problem 8.6-4(b) (c) (d) (b)...
-
In Question 3, suppose that the use of the network follows a pattern in which the number of hours in use is 2,920 for Year 1, 2,190 for Year 2, and 1,460 for Year 3. All other conditions are the...
-
State whether each of the following will increase or decrease the power of a one-way between-subjects ANOVA. (a) The effect size increases. (b) Mean square error decreases. (c) Mean square between...
-
Sketch the graph of each line. y=5 --4-3-2-1 15 4 3 19 44 61 1 2 3 to 56x
-
Treatments of a 1, 3-diketone such as 2,4-pentanedione with base does not give an aldol condensation product. Explain.
-
What product would expect to obtain from base treatment of 1,6-cyclo-decacedione? Base 1,6-Cyclodecanedione
-
Show the products you would except to obtain by claisen condensation of the following esters: (a) (CH3)2CHCH2COEt (b) Ethyl phenyl acetate (c) Ethyl cyclohexylacetate
-
Palisade Creek Co. is a merchandising business that uses the perpetual inventory system. The account balances for Palisade Creek Co. as of May 1, 2019 (unless otherwise indicated), are as follows:...
-
1-When accounting for an acquisition, goodwill is the difference between what two things? 2- What factors should be considered when deciding whether an acquisition should be financed with cash or...
-
What is the main friction Fluidity aims to address? REAL STATE

Study smarter with the SolutionInn App


