The syntheses shown here are unlikely to occur as written. What is wrong witheach? 1. Mg 2.
Question:
The syntheses shown here are unlikely to occur as written. What is wrong witheach?
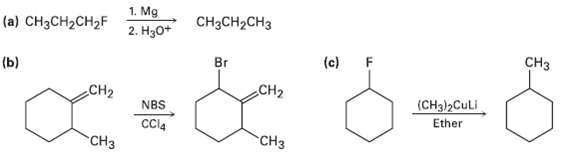
Transcribed Image Text:
1. Mg 2. Нзо* CH3CH2CH3 (a) CH3CH2CH2F Cнз (c) F CH2 Br (ь) CH2 (CH3)2CULI Ether NBS CCl4 "СНз CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
a Fluoroalkanes dont usually form Grignard reagen...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The reactions shown below are unlikely to occur as written. Tell what is wrong with each, and predict the actualproduct. OCCH3)3 . H2CH Br (a) CHCH-CH (CH]l3c (b) Na* - CI LCH3 LCH3 (c) Socil,...
-
Here are five questions written by one of your systems analysis team members. Her interviewee is the local manager of LOWCO, an outlet of a national discount chain, who has asked you to work on a...
-
What is wrong with these synthesesexplain. 1) NaNH2, NH, (1) C=CCH3 a) CH3C=CH 2) -Br CH;CH,-NH, I b) CH;CH,I + NH3 CI + Br OCH, CH, + CH,O d) . .. Br + HBr e) CH3 CH3 H,SO, f)
-
8 for 0 < < 6 for 6
-
Refer to the Snoey Software Company case. Design a spreadsheet that will determine the annual profit when the prices for the Educational, Large-Scale, and Professional versions are $100, $300, and...
-
The potential at the center of a 4.0-cm-diameter copper sphere is 500 V, relative to V = 0 V at infinity. How much excess charge is on the sphere?
-
Jane Moreno and Javier Sanchez combine their resources and open a Latin American tapas restaurant. Moreno puts in $15,000 cash and kitchen equipment with a book value of $6,000, accumulated...
-
Briefly describe some of the similarities and differences between U.S. GAAP and iGAAP with respect to the accounting for leases.
-
Please answer the three parts of the question @ https://www.chegg.com/homework-help/-13th-edition-chapter-4-problem-2QE-solution-9781259741012
-
Sharp Company manufactures a product for which the following standards have been set: Standard Quantity or Hours Standard Price or Rate Standard Cost Direct materials 3 feet $ 11 per foot $ 33 Direct...
-
How would you carry out the followingsyntheses? Cyclohexene Cyclohexanol Cyclohexane ~/~/al
-
Why do you suppose it?s not possible to prepare a Grignard reagent from a bromo alcohol such as 4-bromo-1-pentanol? Give another example of a molecule that is unlikely to form a Grignard reagent....
-
What is competition like in the luxury goods industry? What competitive forces seem to have the greatest effect on industry attractiveness? What are the competitive weapons that rivals are using to...
-
Consider the expression timing is everything in relation to the building of the TOMS brand. Besides the influence of recovering economic conditions and the increased affluence of potential customers,...
-
What is corporate strategy and why is it important? Choose a company with which you are familiar, and evaluate its corporate strategy, especially in regards to financial strategies. What are some...
-
Assignment Tasks: Review the following situations and for each pay period determine the employee's net pay by calculating what earnings & benefits are subject to Income Tax, Canada / Quebec Pension...
-
sample letter for signature change on bank accounts for principals of school
-
Use Excelshowing all work and formulasto complete the following: Prepare a flexible budget. Compute the sales volume variance and the variable cost volume variances based on a comparison between...
-
Explain the difference between active and passive listening.
-
1) The government decided to reduce taxes on fast-food to increase revenue. The government assumes that fast-food products have a) An inelastic demand b) An elastic demand c) A demand curve that is...
-
List the following gas-phase ion pairs in order of the quantity of energy released when they form from separated gas-phase ions. List the pair that releases the least energy first. Na + F , Mg 2+ F ...
-
Draw a reaction-energy diagram for the following reaction: CH3 + Cl2 CH3Cl + Cl The activation energy is 4 kJ mol (1 kcal mol), and the overall Ho for the reaction is -109kJ/mole (-26 kcal/mol) (b)...
-
The bromination of methane proceeds through the following steps: (a) Draw a complete reaction-energy diagram for this reaction. (b) Label the rate-limiting step. (c) Draw the structure of each...
-
(a) Using the BDEs in Table 4-2 (p. 143), compute the value of Ho for each step in the iodination of methane. (b) Compute the overall value of Ho for iodination. (c) Suggest two reasons why iodine...
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


