Question: The time-averaged potential of a neutral hydrogen atom is given by where q is the magnitude of the electronic charge, and ? ?1 = ?
The time-averaged potential of a neutral hydrogen atom is given by
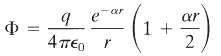
where q is the magnitude of the electronic charge, and ??1 = ?0/2, ?0 being the Bohr radius. Find the distribution of charge (both continuous and discrete) that will give this potential and interpret your result physically.
-exr ar 4 2.
Step by Step Solution
3.45 Rating (171 Votes )
There are 3 Steps involved in it
We are given the time average potential for the Hydrogen atom Since this potential f... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
44-P-E-E-S (180).docx
120 KBs Word File


