The wave nature of particles results in the quantum mechanical situation that a particle confined in a
Question:
The wave nature of particles results in the quantum mechanical situation that a particle confined in a box can assume only wavelengths that result in standing waves in the box, with nodes at the box walls.(a) Show that an electron confined in a one dimensional box of length L will have energy levels given by(b) If a hydrogen atom is modeled as a one-dimensional box with length equal to the Bohr radius, what is the energy (in electron volts) of the lowest energy level of theelectron?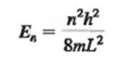
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





