Treatment of a cyclic ketone with diazomethane is a method for accomplishing a ring-expansion reaction. For example,
Question:
Treatment of a cyclic ketone with diazomethane is a method for accomplishing a ring-expansion reaction. For example, treatment of Cyclohexanone with diazomethane yields Cycloheptanone. Propose amechanism.
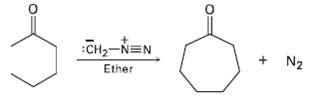
Transcribed Image Text:
Сна-NEN Ether N2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
CH NEN 0 CHNEN 18 ...View the full answer

Answered By

Douglas Makokha
Unlock Academic Success with Dedicated Tutoring and Expert Writing Support!
Are you ready to excel in your academics? Look no further! As a passionate tutor, I believe that dedication and hard work are the keys to achieving outstanding results. When it comes to academics, I strive to provide nothing but the best for every student I encounter.
With a relentless thirst for knowledge, I have extensively researched numerous subjects and topics, equipping myself with a treasure trove of answers to tackle any question that comes my way. With four years of invaluable experience, I have mastered the art of unraveling even the most intricate problems. Collaborating with esteemed writers has granted me exclusive access to the trade secrets utilized by the industry's top professionals.
Allow me the pleasure of assisting you with your writing assignments. I thrive on challenges and will guide you through any obstacles you may face. Together, we will unlock your academic potential and pave the way for your success.
4.90+
62+ Reviews
349+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a mechanism for the acid-catalyzed reaction of cyclohexanone with pyrrolidine.
-
Propose a mechanism for the reaction of cyclohexyl methyl ketone with excess bromine in the presence of sodium hydroxide.
-
A standard synthetic sequence for building a six-membered cyclic ketone onto an existing aromatic ring is shown in outline as follows. Specify the reagents necessary for each step. CCH2CH2COH CH...
-
Governmental Funds Statement of Revenues Expenditures and Changes in Fund Balance. You have recently started working as the controller for a small county. The county is preparing its financial...
-
How can you determine when it is appropriate to send an e-mail, a memo, or a letter?
-
In the analysis of the hypothetical reference of Fig. 4.44, the current I 1 was assumed proportional to temperature. Assume instead that this current is derived from a diffused resistor, and thus has...
-
8. Suppose that f : R ---+ R satisfies f(x + y) = f(x) + f(y) for each x, y E R. (a) Show that f(nx) = nf(x) for all x E Rand n E Z. (b) Prove that f(qx) = qf(x) for all x E Rand q E Q. (c) Prove...
-
Plantcity is a large nursery and retail store specializing in house and garden plants and supplies. Jean Raouth, the assistant manager, is in the process of budgeting monthly supplies expense for...
-
Other things held constant, (1) if the expected inflation rate decreases, and (2) investors become more risk averse, the Security Market Line would shift Down and have steeper slope. Up and have less...
-
Green Manufacturing is a traditional manufacturing company located in the midwestern United States. The companys operations manager is developing a strategy to become more CSR-oriented. In an effort...
-
The Favorskii reaction involves treatment of an ?-bromo ketone with base to yield a ring-contracted product. For example, reaction of 2-bromocyclo-hexanone with aqueous NaOH yields...
-
Ketones react slowly with benzeneselenenyl chloride in the presence of HCl to yield ?-phenylselcno ketones. Propose a mechanism for this acid-catalyzed a-substitution reaction. CeHgSeCi Se-C6H5
-
Rollerblade Corp. has budgeted fixed overhead costs of $50,000 per month plus a variable rate of $4 per direct labor hour. The total factory overhead rate is $6. Actual factory overhead in October is...
-
Using a ruler and set squares only, construct the following shapes: a. b. c. d. 5cm 5cm
-
The marketing department has just forecast that 10,000 units of item 778 will be ordered in the next fiscal year. Based on the marketing department's forecast and noting that the seasonal relative...
-
Following are interaction plots for three data sets. Which data set has the largest interactions? Which has the smallest? A B C
-
From your local chamber of commerce, obtain the population figures for your city for the years \(1980,1990,2000\), and 2010. Find the rate of growth for each period. Forecast the population of your...
-
A mass \(m\) is attached at the midpoint of a stretched wire of area of cross-section \(A\), length \(l\), and Young's modulus \(E\) as shown in Fig. 13.29. If the initial tension in the wire is...
-
Divide into groups of 56 members. LO.1
-
Match the following. Answers may be used more than once: Measurement Method A. Amortized cost B. Equity method C. Acquisition method and consolidation D. Fair value method Reporting Method 1. Less...
-
Determine the molecular geometry of CBr 4 . a) Linear b) Trigonal planar c) Tetrahedral d) Trigonal pyramidal
-
Explain why the differences between the first and second pKa values of the dicarboxylic acids become smaller as the lengths of their carbon chains increase (Table 20.3). TABLE 20.3 pK, Values of Some...
-
What is the pH of a solution containing a buffer consisting of acetic acid and sodium acetate in which the actual [acetic acid]/[sodium acetate] ratio is (a) 1/3? (b) 3? (c) 1?
-
Show why HBr is a stronger acid in acetic acid solvent than it is in water.
-
20 On January 1, Year 1, X Company purchased equipment for $80,000. The company estimates that the equipment will have a useful life of 10 years and a residual value of $5,000. X Company depreciates...
-
Discuss why it is important for company managers to understand and use social capital knowledge to help build social ties among their skilled knowledge workers so they can build employee loyalty...
-
Kate lives in a house close to a local university, and she traditionally has rented a garage apartment in the back of her property to students for $750 per month. Kate wants to transfer the title to...

Study smarter with the SolutionInn App


