Use the infrared absorption spectrum of HCl in Figure to obtain (a) The characteristic rotational energy E
Question:
Use the infrared absorption spectrum of HCl in Figure to obtain
(a) The characteristic rotational energy E0r (in eV)
(b) The vibrational frequency f and the vibrational energy hf (in eV).
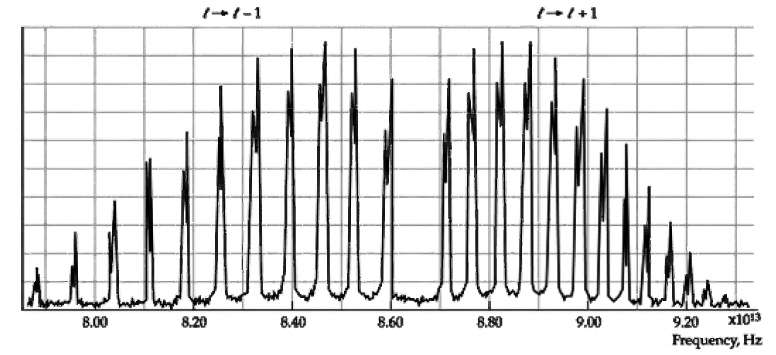
Transcribed Image Text:
t-l+1 х1013 8.40 8.80 8.00 8.20 8.60 9.00 9.20 Frequency, Hz
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (22 reviews)
a Using the equation I h 4 2 f and Equations 3...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:
Students also viewed these Modern Physics questions
-
The infrared absorption spectrum of 1H35Cl has its strongest band at 8.65 x 1013Hz. For this molecule, D0 = 4.43 eV. (a) Find De for 1H35Cl. (b) Find D0 for 2H35Cl
-
A simulated infrared absorption spectrum of a gas phase organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this...
-
(a) The infrared absorption spectrum of 1H35Cl has its strongest band at 8.65 1013 Hz. Calculate the force constant of the bond in this molecule. (b) Find the approximate zero-point vibrational...
-
Shemekia applied to a college that uses multiple regression to select students. This college only considers students whose predicted firstyear GPA is 3.0 or higher. Shemekias predicted GPA was 2.9...
-
From the following income statement (Figure 21.14), balance sheet (Figure 21.15), and additional data for Cygan Company, prepare a statement of cash flows using the indirect method. Additional Data...
-
Assume that the cost, in dollars, of producing x gaming devices is given by C(x) = -0.04x 2 + 80x + 75. (a) Find the marginal cost function, C(x). (b) Find and interpret the marginal cost when x =...
-
Halloween candy rankings. The website FiveThirtyEight. com published a story titled The Ultimate Halloween Candy Power Ranking (October 2017). The rankings were based on data collected from an online...
-
The following costs result from the production and sale of 12,000 CD sets manufactured by Gilmore Company for the year ended December 31, 2013. The CD sets sell for $ 18 each. The company has a 25%...
-
QUESTION 24 The statute of frauds applies only to formal contracts. executory contracts. o informal contracts. executed contracts. QUESTION 25 A guardian is a court-appointed adult who can enter into...
-
A decision tree is a schematic representation of the alternatives available to a decision maker and their possible consequences (Stevenson, 2021). Dream Games is an organization dedicated to the...
-
Find the dependence of the force on separation distance between two polar molecules.
-
For a molecule such as CO, which has a permanent electric dipole moment, radiative transitions obeying the selection rule = 1 between two rotational energy levels of the same vibrational level are...
-
During 2016, Tom sold Sears stock for $10,000. The stock was purchased 4 years ago for $13,000. Tom also sold Ford Motor Company bonds for $35,000. The bonds were purchased 2 months ago for $30,000....
-
In the exchange lemma for the scheduling problem, we say that the first event to finish a* in a given time period [i,j] is always part of the optimal solution for that same time period. To argue...
-
4 10 points Company's year-end is December 31. Calculate depreciation for each year of the machine's estimated useful life under each of the following methods: (Do not round intermediate...
-
3. The walls of an oven are made from steel sheets with insulating board between them of thermal conductivity 0.18 J m-1 s -1 C-1 . If the maximum internal temperature in the oven is 300C and the...
-
Egyptian Spa produces two different spa products: Relax and Refresh. The company uses three operations to manufacture the products: mixing, blending, and packaging. Because of the materials used,...
-
Part A At a given instant A has the motion shown in (Figure 1). Determine the acceleration of B at this instant. Express your answer in feet per second squared to three significant figures. Enter...
-
George Vorman was being recruited by the National Aeronautics and Space Administration (NASA) as a defense intelligence coordinator; that position involved access to classified intelligence and...
-
The power company must generate 100 kW in order to supply an industrial load with 94 kW through a transmission line with 0.09 resistance. If the load power factor is 0.83 lagging, find the...
-
In the circuits of Figs. 5-4 and 5-5 let R 1 = 1 k and R 2 = 5 k. Find the gains G + = v 2 /v s in Fig. 5-4 and G = v 2 /v s in Fig. 5-5 for k = 1, 2, 4, 6, 8, 10, 100, 1000, and . Compare the...
-
What are Eo and Bo 2.00 m from a 95- W light source? Assume the bulb emits radiation of a single frequency uniformly in all directions.
-
Estimate the rms electric field in the sunlight that hits Mars, knowing that the Earth receives about 1350 W / m2 and that Mars is 1.52 times farther from the Sum (on average) than is the Earth.
-
At a given instant in time, a traveling EM waves is noted to have its maximum magnetic field pointing west and its maximum electric field pointing south. In which direction is the wave traveling? If...
-
8 Required information Part 1 of 2 Use the following information for the Exercises below. [The following information applies to the questions displayed below. 1.11 points Hart Company made 3,500...
-
The Russell Company provides the following standard cost data per unit of product: During the period, the company produced and sold 22,000 units incurring the following costs: 1. The direct labor...
-
estion 20 Increase in gross fixed assets is $700 Depreciation expense is $250 Increase in current assets is $600 Increase in accounts payable and accruals is $250 Operating cash flow is $1,200 What...

Study smarter with the SolutionInn App


