A simulated infrared absorption spectrum of a gas phase organic compound is shown in the following figure.
Question:
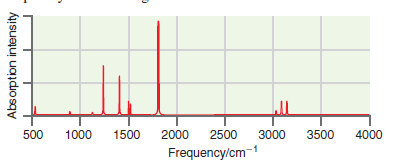
Transcribed Image Text:
1000 2000 500 1500 2500 3000 3500 4000 Frequency/cm-1 Absorption intensity
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 41% (12 reviews)
The peaks near 3100 cm 1 are indicative of CH stretching modes the peak ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A simulated infrared absorption spectrum of a gas-phase organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this...
-
A simulated infrared absorption spectrum of a gas-phase organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this...
-
A simulated infrared absorption spectrum of a gas-phase organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this...
-
According to the Statute of Frauds, in order to be legally enforceable, a contract must be in writing, name the contracting parties, identify the subject matter of the contract, and Be for a legal...
-
In Exercises 1-4, use the half-angle formulas to determine the exact values of the sine, cosine, and tangent of the angle. 1. 75 2. 165 3. 112 30o 4. 67 330o
-
QUESTION THREE INVENTORY CONTROL GreenLantern,pub&grillestimatesthatitwillsell10000beersperyearwhichitwill purchasefromadistributorinLuanshya.AbeercostsK18.00topurchaseandK2.00in...
-
Describe the relationship among the production plan, the master production schedule, and the material requirements plan. LO.1
-
Assume the same information as E14-9 and that Steffi Graf Inc. reports net income in 2008 of $120,000 and in 2009 of $140,000. Total holding gains (including any realized holding gain or loss)...
-
E rmentSessionLocatora &inprogresse false Calculator - Print item The total factory overhead for Big Light Company is budgeted for the year at $924,480. Big Light manufactures two different products...
-
Which one of the following items is remeasured using the current exchange rate under the temporal method? a. Accounts payable. b. Dividends declared. c. Additional paid-in capital. d. Amortization...
-
Purification of water for drinking using UV light is a viable way to provide potable water in many areas of the world. Experimentally, the decrease in UV light of wavelength 250 nm follows the...
-
The molecules 16 O 12 C 32 S and 16 O 12 C 34 S have values for h/8Ï 2 I of 6081.490 Ã 10 6 s 1 and 5932.816 Ã 10 6 s 1 , respectively. Calculate the CO and CS bond distances. The...
-
Explain why the restriction n -1 is necessary in the rule [x" d x" dx = xn+1 n+ 1 + C.
-
1. A concise introduction of the brand, including but not limited to a brief history, location information, size of the business, product/service offering, and so on.Give brief explanation. 2. Which...
-
A wood frame structure as shown to the right. The framingconsists of 2x6 studs, a single 2x6 bottom plate, two 2x6 topplates and a 2x10 joist. The studs are spaced at 16 in. on centerand sheathed...
-
In your reflection journal please list your order - 'most efficient' mediums at the top, 'least efficient' at the bottom. (for eg. social media, display ads, etc)Then, in five hundred words or more,...
-
Energy prices and global warming are discussed daily in the news as environmental impact of e-waste is just beginning to be recognized. Sustainability and corporate social responsibility need to be...
-
3. A Channel section is connected to a 10mm gusset plate with 20mm- diameter bolts as shown in the figure. The connecting member is subjected to dead load and live load only. The pitch distance,...
-
The length of a rectangle is 3 in. more than its width. If the length were decreased by 2 in. and the width were increased by 1 in., the perimeter of the resulting rectangle would be 24 in. Find the...
-
TRUE OR FALSE: 1. Banks with a significantly large share of fixed-interest rate home loans are less exposed to interest rate risks. 2. Although Australian banks are pretty big, they are not...
-
Draw an MO energy diagram and determine the bond order for O 2 2 -. Is it diamagnetic or paramagnetic?
-
A polar molecule will exhibit dipoledipole forces. Which molecules will show dipoledipole forces? a) CHF 3 b) OCS c) H 2 S
-
Calculate the energy in kJ necessary to vaporize 100 g of water at its boiling point.
-
Hite corporation intends to issue $160,000 of 5% convertible bonds with a conversion price of $40 per share. The company has 40,000 shares of common stock outstanding and expects to earn $600,000...
-
Your portfolio has a beta of 1.17, a standard deviation of 14.3 percent, and an expected return of 12.5 percent. The market return is 11.3 percent and the risk-free rate is 3.1 percent. What is the...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...

Study smarter with the SolutionInn App


