A simulated infrared absorption spectrum of a gas-phase organic compound is shown in the following figure. Use
Question:
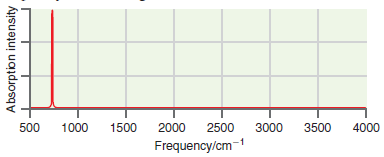
Transcribed Image Text:
500 1000 1500 2000 2500 3000 3500 4000 Frequency/cm-1 Absorption intensity
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
The single peak near 700 cm ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A simulated infrared absorption spectrum of a gas phase organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this...
-
An infrared absorption spectrum of an organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this compound is more likely...
-
An infrared absorption spectrum of an organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this compound is more likely...
-
F udy A big part of communication is sharing personal information with another person. Some information we believe we have the right to be our own and should remain private. However, the degree to...
-
Rewrite tan 3x in terms of tan x.
-
The DuPont analysis of return on equity (ROE) includes all of the following component ratios except: a. Asset turnover. b. Inventory turnover. c. Financial leverage. d. Profit margin.
-
What is closed-loop MRP? LO.1
-
You are the audit senior for the 2015 yearend audit of Vision Quest (VQ), a publicly traded Canadian company that is one of the largest and fastest online vision care providers in the world. Because...
-
A) Bonnie, a widow, elected to receive the proceeds of a $100,000 face value insurance policy on the life of her deceased husband in ten annual installments of $11,900 each including interest...
-
Sunrise Pools and Spas manufactures fiber glass forms for in-ground pools and swim spas for all-season use. The company uses variable costing for internal management reports and absorption costing...
-
The molecules 16 O 12 C 32 S and 16 O 12 C 34 S have values for h/8Ï 2 I of 6081.490 Ã 10 6 s 1 and 5932.816 Ã 10 6 s 1 , respectively. Calculate the CO and CS bond distances. The...
-
The rotational constant for 14 N 2 determined from microwave spectroscopy is 1.99824 cm 1 . Calculate the bond length in 14 N 2 to the maximum number of significant figures consistent with this...
-
The cliff divers of Acapulco push off horizontally from rock platforms about 35m above the water, but they must clear rocky outcrops at water level that extend out into the water 5.0m from the base...
-
Undertake the following and make recommendations for promoting the hotel, bar, restaurants and rooms online: 1. Review regional hotels with bars, a restaurant and limited accommodation and what...
-
W = 235 lb/ft L = 10.5 ft L W The proposed beam for the loading diagram above is a steel 5-in nominal extra strong pipe. What is the maximum bending stress?
-
6. A temporary pedestrian bridge is being designed in Bath for pedestrians to cross the river Avon. A contractor has been employed and the engineering company has decided to support the bridge using...
-
Write the constraints and find the solution for Crypt-arithmetic Problem in Al BASE +BALL B 7 A 4 S8 E 3 GAMES L5 G 1 M 9
-
A well stirred vessel of volume V initially contains fresh water. Dirty water of concentration C_0+cos(wt) (mass/volume) is fed to it at the rate of q (volume/time), where w is the frequency of...
-
In Exercises 7582, express the given function h as a composition of two functions f and g so that h(x) = (f g)(x). h(x) = 1 2x - 3
-
Tarick Toys Company manufactures video game consoles and accounts for product costs using process costing. The following information is available regarding its June inventories. The following...
-
A solution is prepared by dissolving 114 g of glucose (C 6 H 12 O 6 ) in 0.500 kg of water. The final volume of the solution is 590 mL. Calculate each value for this solution: a) molarity b) morality...
-
A solution is prepared from 445 g of ethylene glycol (C 2 H 6 O 2 ) and 500 g of water. This solution represents one that is 50% by volume ethylene glycol. At what temperature will the water in this...
-
A solution contains 0.481 mol of Na 2 SO 4 and 10.0 mol water. Calculate the vapor pressure of the solution at 25C. The vapor pressure of pure water at 25 C is 23.8 torr.
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


