Question: Vapor?liquid equilibrium calculations can sometimes be simplified through the use of a quantity called the relative volatility, which may be defined to terms of the
Vapor?liquid equilibrium calculations can sometimes be simplified through the use of a quantity called the relative volatility, which may be defined to terms of the following depiction of vapor and liquid phases in equilibrium: The relative volatility of species i to species j is. If aij is much greater than 1, species i is much more volatile than species j (i.e., it has a much greater tendency to vaporize at the system temperature and pressure); conversely, if aijij is to 1, the more difficult it is to separate species i from species j by a process such as distillation or partial condensation of a vapor mixture.
(a) Show that the relative volatility of species A to species B, a?AB, equals the ratio of vapor pressures at the system temperature. p*A/p*B if both species obey Raoult?s law and follow ideal gas behavior
(b) Determine the relative volatility of styrene to ethylbenzene at 85?C and the relative volatility of benzene to ethylbenzene at the same temperature. Which pair would you classify as more difficult to separate by distillation?
(c) Show that for a binary mixture of I and j
(d) Apply the equation from part (c) to a benzene?ethylbenzene system at 85?C, using it to estimate the mole fractions of benzene in the vapor phase in equilibrium with Liquids having benzene mole fractions of 0.0, 0.2. 0.4, 0.6, 0.8, and 1.0 then calculate the total system pressure for each.
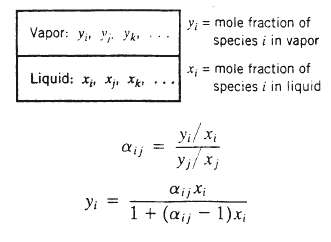
Vapor: y Yk Liquid: X X Xk Yi ij y = mole fraction of species i in vapor x = mole fraction of species i in liquid y/x ij xi 1 + (a, -1)x
Step by Step Solution
3.36 Rating (171 Votes )
There are 3 Steps involved in it
a Raoults law YiPi VP VX PP P a 8 YBXB PBP P3P PB as AB AB ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (314).pdf
180 KBs PDF File
13-E-C-E-C-P (314).docx
120 KBs Word File


