Question: A stage of a separation process is defined as an operation in which components of one or more feed streams divide themselves between two phases,
A stage of a separation process is defined as an operation in which components of one or more feed streams divide themselves between two phases, and the phases are taken off separately. In an ideal stage or equilibrium stage, the effluent (exiting) streams are in equilibrium with each other. Distillation columns often consist of a series of vertically distributed stages. Vapor flow upward and liquid flows downward between adjacent stages; some of the liquid fed to each stage vaporizes, and some of the vapor fed to each stage condenses. A representation of the top section of a distillation column is shown on the next page. (See Problem 4.26 for a more realistic representation.)
Consider a distillation column operating at 0.4 atm absolute in which benzene and styrene are being separated. A vapor stream containing 65 mole% benzene and 35 mole% styrene enters stage 1 at a rate of 200 mol/h and liquid containing 55 mole% benzene and 45 mole% styrene leaves this stage at a rate of 150 mol/h. You may assume (I) the stages are ideal, (2) Raoult's law can be used to relate the compositions of the streams leaving each stage, and (3) the total vapor and liquid molar flow rates do not change by a significant amount from one stage to the next.
(a) How would you expect the mole fraction of benzene in the liquid to vary from one stage to another, beginning with stage 1 and moving up the column? In light of your answer and considering that the pressure remains essentially constant from one stage to another, how would you then expect the temperature to vary at progressively higher stages? Briefly explain.
(b) Estimate the temperature at stage 1 and the compositions of the vapor stream leaving this stage and the liquid stream entering it. Then repeat these calculations for stage 2.
(c) Describe how you would calculate the number of ideal stages required to reduce the styrene content of the vapor to less than 5mole%.
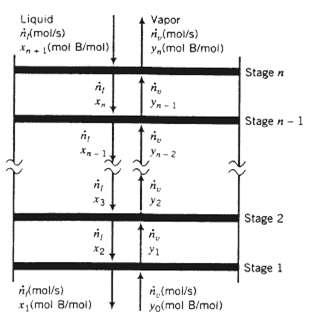
Liquid A (mol/s) (mol B/mol) , Xn XH /mol/s) (mol 8/mol) Vapor ri. (mol/s) y(mol B/mol) A x2 Yn-1 dis Y2 Yn-2 n (mol/s) yo(mol B/mol) Stage n Stage n-1 Stage 2 Stage 1
Step by Step Solution
3.53 Rating (167 Votes )
There are 3 Steps involved in it
a Since benzene is more volatile the fraction of benzene will increase moving up the column For id... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (315).pdf
180 KBs PDF File
13-E-C-E-C-P (315).docx
120 KBs Word File


