Question: We saw in Section 17.4 that ketones react with NaBH 4 to yield alcohols. We?ll also see in Section 22.3 that ketones react with Br
We saw in Section 17.4 that ketones react with NaBH4 to yield alcohols. We?ll also see in Section 22.3 that ketones react with Br2 to yield a-bromo ketones. Perhaps surprisingly, treatment with NaBH4 of the a-bromo ketone from acetophenone yields an epoxide rather than a bromo alcohol. Show the structure of the epoxide, and explain its formation.
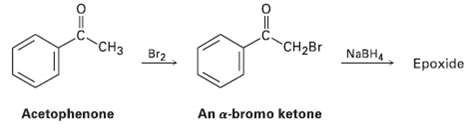
CH2BR NABH ide CH3 Br2 An a-bromo ketone Acetophenone
Step by Step Solution
3.28 Rating (166 Votes )
There are 3 Steps involved in it
H CHBr addition of hydride to the ketone CH Br H di... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-E (97).docx
120 KBs Word File


