Well see in the next chapter that alkyl halides react with nucleophiles to give substitution products by
Question:
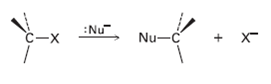
We’ll see in the next chapter that alkyl halides react with nucleophiles to give substitution products by a mechanism that involves inversion of stereochemistry at carbon: Draw the reaction of (S)-2-bromobutane with HS– ion to yield 2-butanethiol, CH3CH2CH (SH) CH3. What is the stereochemistry of the product?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





