What alkyl halides would you use to prepare the following ketones by an acetoacetic ester synthesis? CH
Question:
What alkyl halides would you use to prepare the following ketones by an acetoacetic ester synthesis?
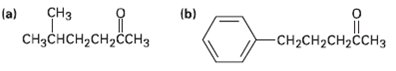
Transcribed Image Text:
CHз (a) (b) CHзснсH,сH,H3 -CH-CH2CH2CCHЗ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
As in the malonic ester synthesis you should identify the stru...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What alkyl halides would you use to prepare the following -amine acids by the amidomalonate method? (a) Leucine (b) Histidine (c) Tryptophan (d) Methionine
-
What reagents would you use to prepare the following compounds? a. b. CH CCH CH:C 0 CH3CCH-CH-CH (COCH-CH3 )2
-
What alkyl halides would you utilize to synthesize the following compounds, using the organoborane shown? a. b. c.
-
What is the value of a 13% coupon bond that is otherwise identical to the bond described in Part D? Would we now have a discount or a premium bond?
-
List the guidelines for effective headings.
-
1. What would you have done if you were the employer who saw this news article? Why? 2. Does the courts decision surprise you? Explain. 3. If you were the employer, what would you do if the employee...
-
8. Assume a calendar-year corporation has a deficit in current E&P of ($100) and positive accumulated E&P of $100. Under this circumstance, a cash distribution of $100 to the corporations sole...
-
Regis Clothiers can borrow from its bank at 17 percent to take a cash discount. The terms of the cash discount are 3/19, net 45. Should the firm borrow the funds?
-
Multiple Choice $2,173,869 $2,018,592 $2,282,562 $1,766,268 $1,850,376
-
If 2 drivers are randomly selected without replacement, find the probability that they both used seatbelts. Use the data in the accompanying table and express all results in decimal form. (The data...
-
How could you use a malonic ester synthesis to prepare the following compound?
-
Which of the following compounds cannot be prepared by an acetoacetic ester synthesis? Explain. (a) Phenyl acetone (b) Acetophenone (c) 3, 3-Dimethyl-2-butanone
-
In your working papers, indicate whether each of the following businesses is a service business, a merchandising business, or a manufacturing business. 1. International Business Machines (IBM) 2. Gap...
-
Company, a soft-drink vendor, has created a table of costs for three stocking decisions for three different states of nature: Alternatives States of Nature Low Demand Medium Demand High Demand Large...
-
utilizes a project scheduling software application to develop a project schedule for a construction project.
-
Kimi is a server or restaurant and relies on tips from a customers to make a living. She doesn't really enjoy her job and frequently think about quitting because she is constantly having 2% a happy...
-
What represents revenues (Inflow) and expenses (Outflow) for a healthcare organization? Your paper should include information on sources of healthcare revenue (governmental and private payers), how...
-
case study analysis should be a thoughtful write up including: 1.a brief case analysis 2.key questions and answers from the case from your unique perspective 3.a summary and/or recommendations...
-
How does a business evaluate its strategic choices? AppendixLO1
-
If you want to solve a minimization problem by applying the geometric method to the dual problem, how many variables and problem constraints must be in the original problem?
-
What is hybridization? Why is hybridization necessary in valence bond theory?
-
Rank the following compounds in order of increasing reactivity (least reactive first) in an SN1 solvolysis reaction in aqueous acetone. Explain your answers. (The structure of tert-cumyl chloride is...
-
Explain why compound A reacts faster than compound B when they undergo solvolysis in aqueous acetone. CH C-Cl CH3 CH CH
-
A hydrocarbon A, C9H12, is treated with A-bromosuccinimide in CCl4 in the presence of peroxides to give a compound B, C9H11Br. Compound B undergoes rapid solvolysis in aqueous acetone to give an...
-
Duncan Inc. issued 500, $1,200, 8%, 25 year bonds on January 1, 2020, at 102. Interest is payable on January 1. Duncan uses straight-line amortization for bond discounts or premiums. INSTRUCTIONS:...
-
WISE-HOLLAND CORPORATION On June 15, 2013, Marianne Wise and Dory Holland came to your office for an initial meeting. The primary purpose of the meeting was to discuss Wise-Holland Corporation's tax...
-
Stock in ABC has a beta of 0.9. The market risk premium is 8%, and T-bills are currently yielding 5%. The company's most recent dividend is $1.60 per share, and dividends are expected to grow at a 6%...

Study smarter with the SolutionInn App


