What cathode potential (versus S.H.E.) is required to reduce 99.99% of Cd(II) from a solution containing 0.10M
Question:
What cathode potential (versus S.H.E.) is required to reduce 99.99% of Cd(II) from a solution containing 0.10M Cd(II) in 1.0 M ammonia if there is negligible current? Consider the following reactions and assume that nearly all Cd(II) is in the form Cd(NH3)42+.
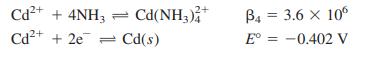
Transcribed Image Text:
Cd2+ + 4NH3 Cd(NH,)* 2+ B4 = 3.6 x 10 Cd2+ + 2e = Cd(s) E° = -0.402 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 84% (13 reviews)
When 9999 of CdII is reduced the form...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
A proton has an initial speed of 5.5 105 m/s. (a) What potential difference is required to bring the proton to rest? (b) What potential difference is required to reduce the initial speed of the...
-
Ti 3+ is to be generated in 0.10 M HClO 4 solution for coulometric reduction of azobenzene. At the counter electrode, water is oxidized, and O 2 is liberated at a pressure of 0.20 bar. Both...
-
Electrogravimetric analysis with control of the cathode potential is proposed as a means for separating Bi3+ and Sn2+ in a solution that is 0.250 M in each ion and buffered to pH 1.95. (a) Calculate...
-
Suppose in a given area there are three power plants, each of which emits SO 2 with different intensities. The abatement cost functions for each firm j are: (a) Set up the conditions for the socially...
-
LaPorta Company and Lott Corporation, two corporations of roughly the same size, are both involved in the manufacture of in-line skates. Each company depreciates its plant assets using the...
-
AP Brady Company entered into these transactions during May 2025, its first month of operations. 1. Stockholders invested \(\$ 40,000\) in the business in exchange for common stock of the company. 2....
-
Assume you have collected the following data for your project. Its budget is $75,000 and it is expected to last four months. After two months, you have calculated the following information about the...
-
The situations presented here are independent of each other. Instructions For each situation, prepare the appropriate journal entry for the redemption of the bonds. (a) Martha Corporation retired...
-
The antouet of money made, er yeur prefit is: (Found to the noarest dotar)
-
On January 2, 2021, Ms. Sherry Kantor moves from London, Ontario, to Thunder Bay, Ontario, in order to begin employment with Northern Enterprises Ltd. (NEL). Her salary for the year was $142,000. NEL...
-
The figure shows the behavior of Pt and Ag cathodes at which reduction of H 3 O + to H 2 (g) occurs. Explain why the two curves are not superimposed. Pt Ag 0.5 -0.2-0.3-0.4-0.6-0.7-0.8-0.9 E(V vs....
-
Electroplating efficiency. Nickel was electrolytically plated onto a carbon electrode from a bath containing 290 g/L NiSO 4 6H 2 O, 30 g/L B(OH)3, and 8 g/L NaCl at - 1.2 V vs. Ag | AgCl. The most...
-
The following is the financial information for Shine Hair Supplies, Ltd.: Additional information: 1. Store equipment was purchased by signing a two-year long-term note payable. 2. Additional bank...
-
Analysis of the Volkswagen Scandal Possible Solutions for Recovery The Volkswagen scandal is a notorious example of how corporations can shape the ethical and political issues of the environment. The...
-
Shelby isn't sure if her forklift can safely handle the pallet she is being asked to move. What can she check to be sure
-
If schedule acceleration increases costs, how could schedule elongation reduce costs? If schedule acceleration increases costs, how could schedule elongation reduce costs? For the same total...
-
Laser Care Hospital is looking to raise tax-exempt municipal funds in the bond market. As an issuer of the bond, which of the following is not a part of the bond process that Laser Care Hospital will...
-
Find the critical value t a/2 corresponding to a 95% confidence level. (13.046, 22.15) X= 17.598 Sx= 16.01712719 n=50
-
Explain the link between the schedule and project costs.
-
How does health insurance risk differ from other types of insurance risk (e.g., automobile or homeowners insurance)? What is the difference between cost sharing and cost shifting? Is retiree health...
-
An unknown sample of Ni 2+ gave a current of 2.36 A in an electrochemical analysis. When 0.500 mL of solution containing 0.028 7 M Ni 2+ was added to 25.0 mL of unknown, the current increased to 3.79...
-
A solution was prepared by mixing 5.00 mL of unknown element X with 2.00 mL of solution containing 4.13 g of standard element S per milliliter, and diluting to 10.0 mL. The signal ratio in atomic...
-
In Figure 5-6, the x-intercept is 2.89 mM and its standard deviation is 0.098 mM. Find the 90% and 99% confidence intervals for the intercept. Figure 5-6
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


