What fragments might you expect in the mass spectra of the followingcompounds? (c) (b)
Question:
What fragments might you expect in the mass spectra of the followingcompounds?
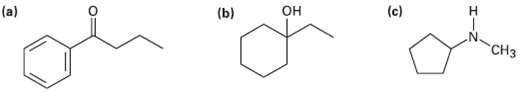
Transcribed Image Text:
(c) (b) он н "СНз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 93% (16 reviews)
a This ketone shows mass spectrum fragments that are due to alpha cleavage and ...View the full answer

Answered By

Sidharth Jain
My name is Sidharth. I completed engineering from National Institute of Technology Durgapur which is one of the top college in India. I am currently working as an Maths Faculty in one of the biggest IITJEE institute in India. Due to my passion in teaching and Maths, I came to this field. I've been teaching for almost 3 years.
Apart from it I also worked as an Expert Answerer on Chegg.com. I have many clients from USA to whom I teach online and help them in their assignments. I worked on many online classes on mymathlab and webassign. I guarantee for grade 'A'.
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The mass spectra of 1-methoxybutane, 2-methoxybutane, and 2-methoxy-2-methylpropane are shown in Figure 13.7. Match the compounds with the spectra. 100 73 80 S 60 57 20 0 10 20 30 40 50 60 70 80 90...
-
What kind of returns might you expect in the stock market? One way to measure how the stock market has performed is to examine the rate of return of the S&P 500 Index. To see historical prices of the...
-
What changes would you expect in the standard deviation for a portfolio of between 4 and 10 stocks, between 10 and 20 stocks, and between 50 and 100 stocks?
-
Shock Electronics sells portable heaters for $35 per unit, and the variable cost to produce them is $22. Mr. Amps estimates that the fixed costs are $97,500. a. Compute the break-even point in units....
-
It has been said that Porter's five-force analysis turns antitrust law on its head. What do you think this means?
-
Exhibit 3.32 presents a statement of cash flows for Walmart for fiscal 2015, 2014, and 2013. This statement matches the Walmart statement of cash flows in Appendix A, and is an expanded version of...
-
Consider the following data collected for a two-way ANOVA: Factor A Factor B Level 1 Level 2 Level 3 Level 1 8 9 10 6 12 28 14 19 24 Level 2 28 31 25 10 21 36 16 19 33 Level 3 39 40 38 33 28 37 23 40...
-
To help finance a major expansion, Castro Chemical Company sold a non callable bond several years ago that now has 20 years to maturity. This bond has a 9.25% annual coupon, paid semiannually, sells...
-
Manny hired his brothers firm to provide accounting services to his business. During the current year, Manny paid his brothers firm $82,000 for services even though other firms were willing to...
-
You decided to run an experiment - improve current CTA on the in-app pricing page (1 experimental variation and one control group). Each month the pricing page is seen by 16,000 users. 800 of those...
-
Assume that you are in a laboratory carrying out the catalytic hydrogenation of cyclohexane to cyclohexane. How could you use a mass spectrometer to determine when the reaction is finished?
-
How might you use IR spectroscopy to distinguish among the three isomers 1 -butyne, 1, 3-hutadiene, arid 2-butyne?
-
What mechanism might be developed to determine whether it is appropriate for government to give a nudge?
-
Find the derivative. 1 f(x)=(4x3+5x)1/3
-
(5.) The Alden Oil Company buys crude vegetable oil. The refining of this oil results in four products, A, B and C, which are liquids and D, which is heavy grease. The cost of the oil refined in 19_9...
-
Derivative of 4 0 0 0 / x + 4 0 + 0 . 1 x
-
covert the polar equation r = 8 3 c o s ( ) - 4 c o s ( ) t o cartesian
-
(2-4)2 <4 Let F (z) = e +4 4
-
Describe the three basic business research designs
-
The following table shows the rates of total return in successive years from 2004 to 2008 for the Sprott Canadian Equity Fund and for the benchmark Toronto Stock Exchange S&P/TSX Composite Index. By...
-
Find the mass of urea (CH 4 N 2 O) needed to prepare 50.0 g of a solution in water in which the mole fraction of urea is 0.0770.
-
Predict the products of the following reactions. ether hexane + 2 Li hexane + 2 Li
-
Show how you would synthesize the following primary alcohols by adding an appropriate Grignard reagent to formaldehyde. (a) (b) (c) CH,OH CH,OH
-
Show two ways you could synthesize each of the following secondary alcohols by adding an appropriate Grignard reagent to an aldehyde. (a) (b) (c)
-
Choose two stocks from the same industry to minimize the influence of other confounding factors. You choose the industry that you are relatively more familiar with, and then estimate the implied...
-
why should Undertake research to review reasons for previous profit or loss?
-
A pension fund's liabilities has a PV01 of $200 million. The plan has $100 billion of assets with a weighted average modified duration of 8. The highest duration bond that the plan can invest in has...

Study smarter with the SolutionInn App


