What product would you obtain from catalytic hydrogenation of the following alkenes? CH2H (a) (b) CH CH
Question:
What product would you obtain from catalytic hydrogenation of the following alkenes?
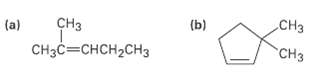
Transcribed Image Text:
сНз Cнзс—снсH2сHз (a) (b) CHз "CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 56% (16 reviews)
Catalytic hydrogenation produces alkanes fr...View the full answer

Answered By

Kennedy Odhiambo
As a professional writer, I have been in the field for over 5 years having worked as a lecture in different tertiary institutions across the world. With this impeccable experience, I assure provision of a good and supporting environment for students to learn.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What Product would you obtain from a base-catalyzed Michael reaction of 3-button-2-one with each of the following nucleophilicdonors? (b) o (a) ELOCH,OET .Et
-
What product would you obtain from a base-catalyzed Michael reaction of 2, 4-pentanedione with each of the following , -unsaturated acceptors? (a) 2-Cyclohexenone (b) Propene nitrile (c) Ethyl...
-
What product would you expect to obtain from catalytic hydrogenation of natural rubber? Would the product be syndiotactic, atactic, or isotactic?
-
Consider the deletion of record 5 from the file as shown below compare the relative merits of the following techniques for implementing the deletion: a. Move record 6 to the space occupied by record...
-
The Fischer Theatre compared attendance at its Saturday and Sunday matinee performances of a major Broadway musical. At = .05, is the Sunday matinee attendance significantly greater than the...
-
What are typical planning analytical procedures related to debt?
-
32. Harmer Inc. is now a successful company. In the early days (before it became profitable), it issued ISOs to its employees. Now Harmer is trying to decide whether to issue NQOs or ISOs to its...
-
Refer to the Real Estate data, which report information on homes sold in Goodyear, Arizona, and the surrounding area. a. Use an appropriate nonparametric test to determine whether there is a...
-
Assume that you are on the financial staff of West Enterprises, and you have collected the following data: (1) The yield to maturity on the companys outstanding 8% annual coupon bonds is 6.0%, and...
-
Rausher Industries began a new product line this year. Management wants a cost report for the current year and a budget for next year. The product requires processing in two departments. Materials...
-
What products would you expect from the following reactions? CH2 (a) + CHCI3 CH (b) Zn(Cu) CH2I2 H2HH
-
What product would you expect from reaction of cis-2-butene with meta-chloro-peroxybenzoic acid? Show the stereo chemistry.
-
An incandescent lightbulb is an inexpensive but highly inefficient device that converts electrical energy into light. It converts about 10 percent of the electrical energy it consumes into light...
-
Among 450 randomly selected drivers in the 16 - 18 age bracket, 374 were in a car crash in the last year. If a driver in that age bracket is randomly selected, what is the approximate probability...
-
Construct a 90% confidence interval for the population standard deviation o at Bank A. Bank A 6.4 6.6 6.7 6.8 7.1 7.2 7.6 7.8 7.8 7.8
-
In 2002, after the accounting deceptions of the management of many multi-million dollar corporations (with Enron being the benchmark name of that time period), the Security and Exchange Commission...
-
1.Deduce the structure of a compound with molecular formula CsH100 that exhibits the following IR, H NMR, and 13C NMR spectra. Data from the mass spectrum are also provided. Mass Spec. Data relative...
-
Transcribed image text: Prots Caco.ch Part 2 Income Statement Med Earningstemet Tante Sheet For the event.com Competence ended The fram C an an dy wana A TO nede ANG ore.com wwwwww og for to...
-
What question should be asked upon the receipt of a responsibility accounting report? LO.1
-
What is the purpose of the journal wizard?
-
Why are intermolecular forces generally much weaker than bonding forces?
-
A widely used undergraduate experiment is the recrystallization of acetanilide from water. Acetanilide (see following structure) is moderately soluble in hot water, but much less soluble in cold...
-
Give the structure of each of the following compounds. (a) Chlorocyclopropane (b) Methylene iodide
-
Acetone (Table 8.2) has a significant dipole moment (2.7 D). Using structures, show the stabilizing interactions to be expected between acetone solvent molecules and (a) A dissolved potassium ion;...
-
The rate of return on Cherry Jalopies, Inc., stock over the last five years was 14 percent, 11 percent, 4 percent, 3 percent, and 7 percent. What is the geometric return for Cherry Jalopies, Inc.?
-
U.S. GAAP specifies all of the following characteristics of variable interest entities except: A. Equity holders hold less than 5% of the entitys voting stock. B. Equity holders do not have voting...
-
Rank the following three stocks by their risk-return relationship, best to worst. Night Ryder has an average return of 10 percent and standard deviation of 27 percent. The average return and standard...

Study smarter with the SolutionInn App


