When a carboxylic acid is dissolved in isotopically labeled water, the label rapidly becomes incorporated into both
Question:
When a carboxylic acid is dissolved in isotopically labeled water, the label rapidly becomes incorporated into both oxygen atoms of the carboxylic acid. Explain.
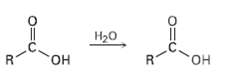
Transcribed Image Text:
Нао R. но, он R.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
R OH HO HO O OH OH R OH HO T The tetrahedral intermedi...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Suppose that the aggregate demand for a depletable resource is given by the following inverse demand function: P= 100 (Q+S) where P is the market price, Q is the amount of depletable resource, and S...
-
a. When a carboxylic acid is dissolved in isotopically labeled water (H2O18), the label is incorporated into both oxygens of the acid. Propose a mechanism to account for this. b. If a carboxylic acid...
-
A carboxylic acid is formed when an α haloketone reacts with hydroxide ion. This reaction is called a Favorskii reaction. Propose a mechanism for the following Favorskii reaction....
-
In a law firm consisting of 20 lawyers, 9 are criminal lawyers, 6 are divorce lawyers, and 4 are both criminal and divorce lawyers. If a lawyer from this firm is chosen at random, what is the...
-
Most workers groan and grumble when they must attend meetings. How can you ensure that the meetings you run are productive?
-
In spherical coordinates, the surface of a solid conducting cone is described by = /4 and a conducting plane by = /2. Each carries a total current I. The current flows as a surface current radially...
-
51. Mackenzie is considering conducting her business, Mac561, as either a single-member LLC or as an S corporation. Assume her marginal ordinary income tax rate is 37 percent, her marginal FICA rate...
-
The Gibson & Zorich Bakery bakes breads and muffins for wholesale to restaurants. The company uses process costing to account for the cost of the breads and muffins that it produces. Raw material...
-
Consider a bank with the following balance sheet. If the bank is holding no exceserves what is the desert Reserves Loans Securities 1,000,000 50, eee Deposits 50e, cee Borrowing see, eee Bank Capital...
-
Rausher Industries began a new product line this year. Management wants a cost report for the current year and a budget for next year. The product requires processing in two departments. Materials...
-
Fats arc biosynthesized from glycerol 3-phosphate and fatty-acyl CoA?s by a reaction sequence that begins with the following step. Show the mechanism of the reaction. CH20-C-R CH- ...
-
When ethyl benzoate is heated in methanol containing a small amount of HC1, methyl benzoate is formed. Propose a mechanism for the reaction.
-
Faello Inc. experienced the following events for the first two years of its operations: 2013: 1. Provided $80,000 of services on account. 2. Provided $22,000 of services and received cash. 3....
-
Complete Exercises 2-B and 2-H in Writing and Analysis in the Law using what you learned in the reading and in the Seminar. Use paragraph form, use complete sentences, and make sure you use proper...
-
What is the value of a stock expected to be in 9 years if the annual dividend is expected to remain unchanged forever at $3.65, the expected rate of return is 6.9% per year, and the next dividend is...
-
Once invested IN a corporation, shareholders want their money out - they want a return on investment! John owns 2 5 % of REFUND CORP INC, which paid out a $ 5 0 , 0 0 0 distribution to him on 1 2 / 3...
-
Worksheet Financial Statement Ratios. Lowe's Companies, Inc Jan 28, 2022 and Jan. 29, 2021 Current Ratio Current Assets / Current Liabilities Acid Test Current Assets Current Liabilities (Cash + ST...
-
3. Peter Senen operates in a JIT manufacturing system. For August, Peter Senen purchased 10,000 units of raw materials at P1.00 per unit on account.What is the The journal entry to record the...
-
Explain why environmental analysis is considered to be an important activity for the strategy formulation process of an organization. AppendixLO1
-
Construct a 4 x 25 design confounded in two blocks of 16 observations each. Outline the analysis of variance for this design.
-
Determine the molecular geometry about each interior atom and sketch each molecule. a. N 2 b. N 2 H 2 (skeletal structure HNNH) c. N 2 H 4 (skeletal structure H 2 NNH 2 )
-
In the preparation of ethynylmagnesium bromide by the transmetallation reaction of Eq. 14.24, ethylmagnesium bromide is added to a large excess of acetylene in THF solution. Two side reactions that...
-
Identify the following compounds from their IR and proton NMR spectra. (a) C4H6O: liberates a gas when treated with C2H5MgBr (b) C5H6O IR: 3300, 2102, 1634 cm-1 NMR: 3.10 (1H, d, J = 2Hz); 3.79 (3H,...
-
Explain how you could distinguish between 1-hexyne and 4-methyl-2-pentyne by 13C NMR.
-
Be prepared to explain the texts comprehensive To illustrate the issues related to interest capitalization, assume that on November 1, 2016, Shalla Company contracted Pfeifer Construction Co. to...
-
On April 1, 2020. Indigo Company received a condemnation award of $473,000 cash as compensation for the forced sale of the company's land and building, which stood in the path of a new state highway....
-
The market price of a stock is $24.55 and it is expected to pay a dividend of $1.44 next year. The required rate of return is 11.23%. What is the expected growth rate of the dividend? Submit Answer...

Study smarter with the SolutionInn App


