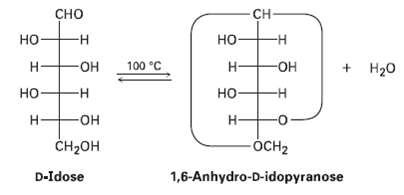
When heated to 100C, n-idose undergoes a reversible loss of water and exists primarily as 1, 6-anhydro-D-idopvranose.
Question:
When heated to 100°C, n-idose undergoes a reversible loss of water and exists primarily as 1, 6-anhydro-D-idopvranose.
(a) Draw D-idose in its pyranose form, showing the more stable chair conformation of the ring.
(b) Which is more stable, ?-D-idopyranose or ?-D-idopyranose? Explain.
(c) Draw 1,6-anhydro-n-idopyranose in its most stable conformation.
(d) When heated to 100 °C under the same conditions as those used for D-idose, 1)-glucose does not lose water and does not exist in a 1, 6-anhydro form. Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





