Which of the following species is likely to be a nucleophile and which anelectrophile? (a) CH3CI (b)
Question:
Which of the following species is likely to be a nucleophile and which anelectrophile?
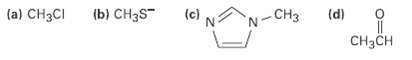
Transcribed Image Text:
(a) CH3CI (b) CH3S" (c) (d) -CH3 CH3CH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
Strategy Keep in mind 1 An electrophile is electronpoor either because it is positively charged bec...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which of the following species is not likely to have a tetrahedral shape? (a) SiBr4, (b) NF4+ (c) SF4, (d) BeCl42-, (e) BF4-, (f) AlCl4-
-
Which of the following species are tetrahedral? SiCl4, SeF4, XeF4, CI4, CdCl42?
-
Which of the following examples is likely to be caused by a somatic mutation? A. A purple flower has a small patch of white tissue. B. One child, in a family of seven, is an albino. C. One apple...
-
Water is an essential resource. For that reason moral considerations exert considerable pressure to assure that everyone has access to at least enough water to survive. Yet it appears that equity and...
-
Three years ago, you purchased 150 shares of IBM stock for $92 a share. Today, you sold your IBM stock for $183 a share. For this problem, ignore commissions that would be charged to buy and sell...
-
The cash account for Pala Medical Co. at June 30, 20Y1, indicated a balance of $166,436. The bank statement indicated a balance of $195,688 on June 30, 20Y1. Comparing the bank statement and the...
-
Alivan Company uses a sales journal, a purchases journal, a cash receipts journal, a cash disbursements journal, and a general journal. The following transactions occur in the month of June. June 1...
-
Sparrow Company uses the retail inventory method to estimate ending inventory and cost of goods sold. Data for the 2006 are as follows: The company records sales net of employee discounts. Discounts...
-
uestin 11 (4 points) is the primary source (or form) of coordination in an organization with a simple undifferentiated structure. Standardization Direct supervision Mutual adjustment Decentralization...
-
Draaksh Corporation sells premium quality wine for $50 per bottle. Its direct materials and direct labour costs are $9 and $6 respectively per bottle. It pays its direct labour employees a wage of...
-
Using a curved fishhook arrow, propose a mechanism for formation of the cyclopentane ring of prostaglandin H2. What kind of reaction isoccurring? CO2H "
-
An electrostatic potential map of boron trifluoride is shown. Is BF3 likely to be a nucleophile or an electrophile? Draw a Lewis structure for BF3, and explain youranswer. BF3
-
Is Mr. Bustamante protected under the ADA?
-
Compare the alternatives that Bergerac is considering for its decision. Include: Comparison of make versus buy option in the type of operation that Bergerac is looking to integrate. You do not need...
-
Let A, B, C and D be non-zero digits, such that CD is a two-digit positive integer. BCD is a three-digit positive integer generated by the digits B, C and D. ABCD is a four-digit positive integer...
-
1.) An aluminum tube is clamped with rigid plates using four bolts as shown. The nut on each bolt is tightened one turn from 'snug'. The thickness of the plate may be considered insignificant in this...
-
4.21 Case Study Competency IV.1RM Determine diagnosis and procedure codes and groupings according to official guidelines. Competency IV.1 Validate assignment of diagnostic and procedural codes and...
-
W.E.B Dubois taught the book called "The State" to his students at Atlanta University. Who wrote this book
-
Beth is an Agile project manager and she wants to create a dashboard for her team. A dashboard can also be known as a what? A. Information radiator B. Kanban board C. Burnup chart D. Queue
-
Banner Company acquires an 80% interest in Roller Company for $640,000 cash on January 1, 2013. The NCI has a fair value of $160,000. Any excess of cost over book value is attributed to goodwill. To...
-
Construct a concept map that embodies the ideas of valence bond theory.
-
5-Chloro-1,3-cyclopentadiene (below) undergoes SN1 solvolysis in the presence of silver ion extremely slowly even though the chlorine is doubly allylic and allylic halides normally ionize readily...
-
Explain the following: (a) Cyclononatetraenyl anion is planar (in spite of the angle strain involved) and appears to be aromatic. (b) Although [16]annulene is not aromatic, it adds two electrons...
-
Furan possesses less aromatic character than benzene as measured by their resonance energies (96 kJ mol-1 for furan; 151 kJ mol-1 for benzene). What reaction have we studied earlier that shows that...
-
The predetermined overhead rate is usually calculated Group of answer choices At the end of each year At the beginning of each month At the beginning of the year At the end of the month
-
ajax county collects property taxes for the cities within the county, Ajax county collected 1000 from citizens in Beatty city that belong to Beatty city what would be the appropriate entries for ajax...
-
Assume that gasoline costs $ 3 . 2 0 per gallon and you plan to keep either car for six years. How many miles per year would you need to drive to make the decision to buy the hybrid worthwhile,...

Study smarter with the SolutionInn App


