Which of these compounds are expected to have an absorption maximum in the region of 200 to
Question:
Which of these compounds are expected to have an absorption maximum in the region of 200 to 400nm in their UV spectra?
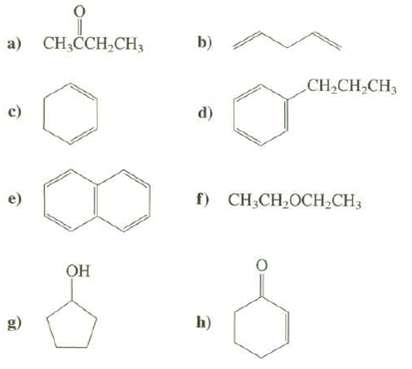
Transcribed Image Text:
а) Cн-СCH,CH, b) CH,CH,CH3 c) d) f) CH,CH,OCH,CH3 e) Он h) g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (18 reviews)
a 2Butanone will show an absorption maximum for its n transition i...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which of these compounds would you expect to have the highest boiling point? Explain. [Section 24.4] CH3CH CH CH OH CHC=CH HCOCH
-
Which of these compounds can be a member of an isomer pair? In each case where isomerism is possible, identify the type or types of isomerism. [Sections 24.2, 24.4] CH2 C C-OH --O NHE Cl (b) CH3CH2CH...
-
Which of these compounds exhibit cis-trans isomerism? Draw both cis-trans isomers when they exist? a) CHCHCH=CHCH CH3 c) CHC=CHCH b) CH3CHCH=CH CI d) CHC=CHCHCH3
-
March 31, 2014, adjusted trial balance for Brenner Climbing Adventures has been alphabetized as follows: Required Journalize the closing entries. No. Account Debit Credit $ 2,600 168 Accumulated...
-
Teloxy Engineering has received a onetime contract to design and build 10,000 units of a new product. During the proposal process, management felt that the new prod-uct could be designed and...
-
The DeWitt Company has found that the rate of change of its average cost for a product is where x is the number of units and cost is in dollars. The average cost of producing 20 units is $40.00. (a)...
-
Ask insiders why things are done that way
-
Use (a) the percentage method and (b) the wage-bracket method to compute the federal income taxes to withhold from the wages or salaries of eachemployee. Amount to Be Withheld Martal No. of...
-
Yadra Ltd owns all of the share capital of Viti Ltd. In relation to the following intragroup transactions, all parts of which are independent unless specified, prepare the consolidation worksheet...
-
Identify the reagents a?c in the following scheme: CH C -CH CH H -CH H.
-
Indicate the types of transitions responsible for the absorptions of these compounds: Apax = 252 nm (e = 20,000) Aas - 325 nm ( = 180) a) A mux = 235 nm (e = 19,000) b) c) Amax = 299 nm ( = 20) d)...
-
Predict the major fragments and their m/z that would appear in the mass spectra of these compounds: a) b) c) - d) ) h) g) i)
-
President Reagan was interested in establishing a defensive wall to blunt the threat of a nuclear missile attack against this country. The plan was commonly known as Star Wars. Assume a defense...
-
7. Chicago Corp. obtained the following information from the Raw Materials Inventory account and purchasing records for the first quarter of the current year: Beginning Raw Materials Ending Raw...
-
Suppose that i t =6% (n=1), and that future short term interest rates (n=1) for the next 3 years (starting next year) are expected to be: 4%, 2%, 2%. Suppose that the liquidity premium is zero for...
-
Mechanical Vibrations HW Use the modal analysis and numerical integration to compute and plot the time response of the system, which has the equations of motion [8 0 01 (1) 48 -12 01(x1 0 0 8 02-12...
-
Submit excel file with graph and exchange rate analysis. FOREIGN EXCHANGE RATESTHE YEN FOR DOLLARS. The Federal Reserve System Web site, www.federalreserve.gov/releases/H10/hist , provides historical...
-
Part 1: There are many types of communication styles used in the workplace. Choose what you think is your leadership style: north, south, east, or west. Click The Leadership Compass Self-Assessment...
-
appreciate the major stages in the development of an effective sport sponsorship or endorsement programme;
-
As of January 1, 2018, Room Designs, Inc. had a balance of $9,900 in Cash, $3,500 in Common Stock, and $6,400 in Retained Earnings. These were the only accounts with balances in the ledger on January...
-
According to the quantity theory of money and prices, how else besides using interest-rate-based policies might central banks be able to generate higher inflation if they really wished to do so?
-
Tetrachloroethene (CCl2==CCl2) does not have a dipole moment. Explain this fact on the basis of the shape of CCl2==CCl2.
-
Why does one expect the cis isomer of an alkene to have a higher boiling point than the trans isomer?
-
Cetylethyldimethylammonium bromide is the common name for a compound with antiseptic properties. Predict its solubility behavior in water and in diethyl ether. Br
-
Use the following information: \ table [ [ Country , \ table [ [ Consumer Prices ] ] , Interest Rates,Current Units ( per US$ ) ] , [ Forecast , 3 - month, 1 - yx Covt Bond,, ] , [ 2 0 2 4 e ,...
-
Year-to-date, Yum Brands had earned a 3.70 percent return. During the same time period, Raytheon earned 4.58 percent and Coca-Cola earned 0.53 percent. If you have a portfolio made up of 40 percent...
-
Rate of Return If State Occurs State of Probability of Economy State of Economy Stock A Stock B Stock C Boom .15 .31 .41 .21 Good .60 .16 .12 .10 Poor .20 .03 .06 .04 Bust .05 .11 .16 .08 a. Your...

Study smarter with the SolutionInn App


