Would you expect the following carboxylic acids to be more acidic or less acidic than benzoic acid?
Question:
Would you expect the following carboxylic acids to be more acidic or less acidic than benzoic acid? Explain. (Reddish brown = Br)
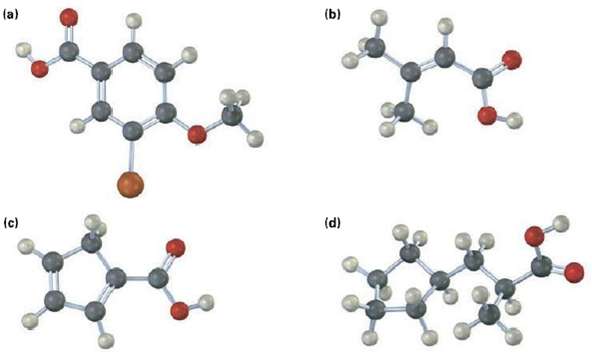
Transcribed Image Text:
(a) (b) (d) (c)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
Br COH b COH CH32N a pBromobenzoic acid is more acidic th...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Would you expect the citric acid cycle to be more or less active when a cell has a high ATP/ADP ratio and a high NADH/NAD+ ratio? Give the reason for your answer.
-
Would you expect the following compound to be aromatic? Explain your answer.
-
Would you expect the substituent? to more closely resemble in its effect on rate and regioselectivity in electrophilic aromatic substitution? Why? N(CH N(CH3)2 or NO2
-
Read the Poem Little Birds Flying and answer the following questions: What it is notifying? To whom it is notifying? How is the work two dimensional? What does it take to realize the project? ...
-
1. What unique challenges did Rande and Jonas face when entering into this partnership? 2. What impact did their friendship seem to have on this partnership? 3. What partner duties did Jonas fail to...
-
What percentage of scores in a normal distribution fall at or above a z score of 1.34?
-
To what extent, if any, is it the governments responsibility to ensure that professional sportsmen and women are appropriate role models for children?
-
The absolute pressure within a 35.0-liter gas cylinder should not exceed 51.0 atm. Suppose the cylinder contains 50.0 mol of a gas. Use the SRK equation of state to calculate the maximum permissible...
-
2 7 . Capital Budgeting Analysis. Wolverine Corp. currently has no existing business in New Zealand but is considering establishing a subsidiary there. The following information has been gathered to...
-
The Fashion Rack has a monthly accounting period. All transactions are recorded in a general journal. Postings are made from the general journal to the accounts receivable ledger, accounts payable...
-
Give IUPAC name for the following carboxylic acids (reddish brown = Br). (a) (b) (d) (c)
-
The following carboxylic acid can?t be prepared from an alkyl halide by either the nitrile hydrolysis route or the Grignard carboxylation route. Explain.
-
With renewable energy set to grow further in the coming years, what more could insurance firms do to encourage take up?
-
Absorption linewidth for an absorbing atomic transition. Consider the curves of power transmission T(w) = exp[-2am(w)L] through an atomic medium with a lorentzian resonant transition, plotted versus...
-
EXAMPLE 05.04 Z Write the force and the couple in the vector form (with rectangular/Cartesian components). Use C = 180 N-m and P = 500 N O INDIVIDUAL Submission (IS12) D x 400 mm B C 300 mm A 400 mm...
-
1.XYZ Corporation budgets factory overhead cost of P500,000 for the coming year. Compute for the overhead cost applied to the job. The following data are available: Budgeted annual overhead for...
-
OP Technologies Manufacturing manufactures small parts and uses an activity-based costing system. Activity Materials Assembling Packaging Est. Indirect Activity Costs $65,000 $242,000 $90,000...
-
3. Solve Example 3.7 (Bergman, Lavine, Incropera, and DeWitt, 6th Ed., pp. 129-132, or 7th Ed., pp. 145-149, or 8th Ed., pp. 134-138), but use the finite difference method. T T = 30C Insulation-...
-
Rights Offerings Again, Inc., is proposing a rights offering. Presently, there are 550,000 shares outstanding at $87 each. There will be 85,000 new shares offered at $81 each. a. What is the new...
-
Find the intercepts and then graph the line. (a) 2x - 3y = 6 (b) 10 - 5x = 2y
-
Polyacetylene is an addition polymer with the structure shown here. Draw the structure of the monomer. H [] C. C H
-
(a) What are the structures of L-(+)-threose and L-(+)-erythrose? (b) What aldotriose would you use to prepare them in a Kiliani-Fischer synthesis?
-
(a) Outline a Kiliani-Fischer synthesis of epimeric aldopentoses starting with D-(-)- erythrose (use Fischer projections). (b) The two epimeric aldopentoses that one obtains are D-(-)-arabinose and...
-
Subjecting D-(-)-threose to a Kiliani-Fischer synthesis yields two other epimeric aldopentoses, D-(+)-xylose and D-(-)-lyxose. D-(+)-Xylose can be oxidized (with nitric acid) to an optically inactive...
-
Explain the following: Understand the PPE acquisition (or investing) cycle and related significant transactions and source documents Understand the relevant assertions/objectives about PPE balances...
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...
-
C: The sor at the poopecin 0ieund to twe oxind places)

Study smarter with the SolutionInn App


