(3.0 mathrm{kmol}) of feed containing (52.0 mathrm{~mol} %) water and (48.0 mathrm{~mol} % mathrm{n})-butanol is charged to...
Question:
\(3.0 \mathrm{kmol}\) of feed containing \(52.0 \mathrm{~mol} \%\) water and \(48.0 \mathrm{~mol} \% \mathrm{n}\)-butanol is charged to the still pot of a simple batch distillation system (Figure 9-1). The final still pot concentration should be \(28.0 \mathrm{~mol} \%\) water. Equilibrium data are in Table 8-5.
Figure 9-1
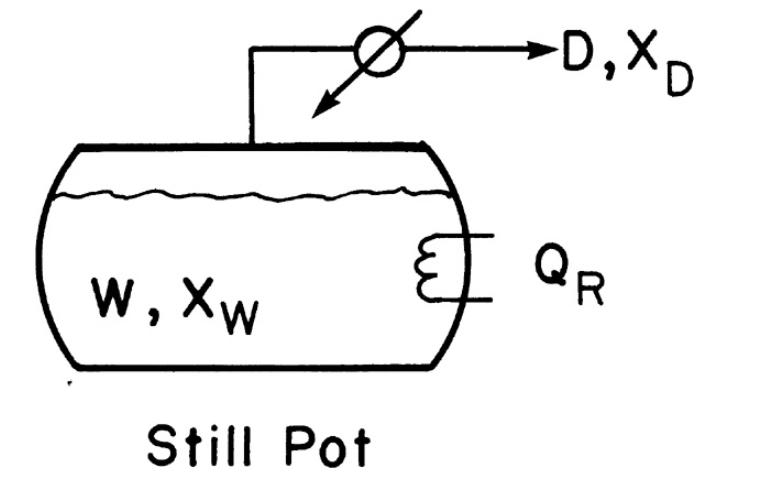
Table 8-5
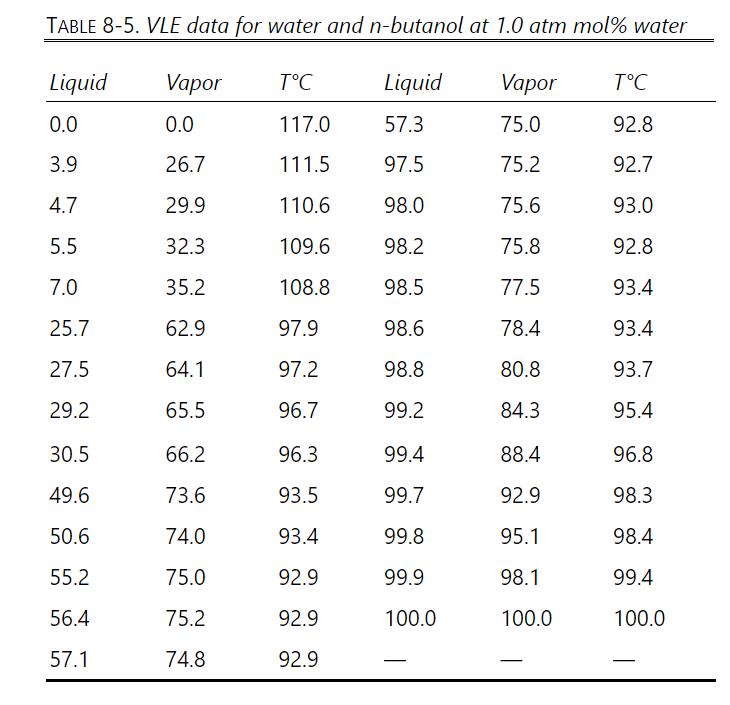
a. Find \(\mathrm{W}_{\text {fin }}\) (kmole), \(\mathrm{D}_{\mathrm{V}, \text { total }}\) (total amount of distillate vapor collected, kmole), and \(\mathrm{y}_{\mathrm{D}, \text { avg }}\) (average mole fraction water in the vapor distillate).
b. After the distillate vapor is condensed in the total condenser, the liquid is sent to a liquid-liquid settler. Find the total amount of each distillate liquid collected, \(\mathrm{D}_{1}\left(\right.\) with \(\left.\mathrm{x}_{\mathrm{D} 1, \text { water }}=0.573\right)\) and \(\mathrm{D}_{2}\) (with \(\left.\mathrm{x}_{\mathrm{D} 2, \text { water }}=0.975\right)\), in kmoles.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





