Question: A 5,000-kg/h aqueous solution of 20 wt% Na2SO4 is fed to an evaporative crystallizer operating at 60?C. Equilibrium data are given in Figure. If 80%
A 5,000-kg/h aqueous solution of 20 wt% Na2SO4 is fed to an evaporative crystallizer operating at 60?C. Equilibrium data are given in Figure. If 80% of the Na2SO4 is to be crystallized, calculate:
(a) The kilograms of water that must be evaporated per hour
(b) The crystallizer pressure intom
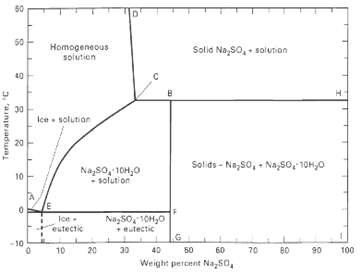
50 50 Homogeneous solution Solid Na,so, soluton 40 30 Ice solunior 20 Solids - Na,50, - Na,So, 10H,0 Nazso, 10H,0 solution 10 Ice eutectic Nay50, 10H,0 + eutectic -10 40 60 10 20 50 70 80 06 100 Weight percent Na,50, 3. ainjeadua
Step by Step Solution
3.30 Rating (159 Votes )
There are 3 Steps involved in it
a From Fig at 60 o C the stable crystals are Na 2 SO 4 with no water of crystallization The solubili... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (159).docx
120 KBs Word File


