Question: An aqueous solution containing 35 wt% MgSO 4 is fed to an evaporative crystallizer operating at 50F. The vapor generated is 20% by mass of
An aqueous solution containing 35 wt% MgSO4 is fed to an evaporative crystallizer operating at 50°F. The vapor generated is 20% by mass of the feed. The solution, which contains 23 wt% MgSO4, and the crystals suspended in it are in equilibrium.
(a) Based on the information in Table 6.5-1, what is the composition of the crystalline product?
(b) For a crystal production rate of 1000 kg/h, estimate (i) the required feed and vapor flow rates in kg/h, and (ii) the rate at which MgSO4 could be recovered from the crystals if all the water is removed from them by drying.
Table 6.5-1
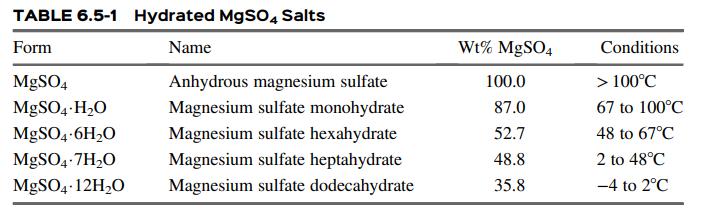
TABLE 6.5-1 Hydrated MgS04 Salts Form Name Wt% MgSO4 Conditions MgSO4 Anhydrous magnesium sulfate 100.0 > 100C MgSO4-H20 Magnesium sulfate monohydrate 87.0 67 to 100C MGSO4-6H20 Magnesium sulfate hexahydrate 52.7 48 to 67C MgSO4-7H,O Magnesium sulfate heptahydrate 48.8 2 to 48C MGSO4 12H2O Magnesium sulfate dodecahydrate 35.8 -4 to 2C
Step by Step Solution
3.57 Rating (168 Votes )
There are 3 Steps involved in it
a Based on the table and the temperature given T10 o C Magnesium sulfate heptahydrate MgSO 4 ... View full answer

Get step-by-step solutions from verified subject matter experts


