Question: A compound A (C6H) undergoes catalytic hydrogenation over Lindlar catalyst to give a compound B, which in turn undergoes ozonolysis followed by workup with aqueous
A compound A (C6H) undergoes catalytic hydrogenation over Lindlar catalyst to give a compound B, which in turn undergoes ozonolysis followed by workup with aqueous H2O2 to yield succinic acid and two equivalents of formic acid. In the absence of a catalyst poison, hydrogenation of A gives hexane. Propose a structure for compound A.
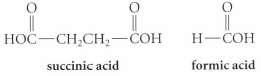
HOC-CH,CH2- - succinic acid formic acid
Step by Step Solution
3.39 Rating (165 Votes )
There are 3 Steps involved in it
The ozonolysis results define compound B as 1 5hexadi... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
902-C-O-O-S (470).docx
120 KBs Word File


