The following structure represents a tetrahedral alkoxide-ion intermediate formed by addition of a nucleophile to a carboxylic
Question:
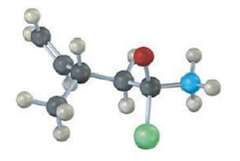
The following structure represents a tetrahedral alkoxide-ion intermediate formed by addition of a nucleophile to a carboxylic acid derivative. Identify the nucleophile, the leaving group, the starting acid derivative, and the ultimate product (yellow-green =Cl):
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (17 reviews)
2 NH3 O H2CCHCHCHCNH3 CH3 HCCHCHCH CH3 NH C 3Methyl4p...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The following structure represents tetrahedral alkoxide ion intermediate formed by addition of a nucleophile to a carboxylic acid derivative. Identify the nucleophile, the leaving group, the starting...
-
The following structure represents an intermediate formed by addition of ester emulation to a second ester molecule. Identify the reactant, the leaving group, and theproduct.
-
The following structure represents the carbocation intermediate formed in the addition reaction of HBr to two different alkenes. Draw the structures ofboth.
-
Explain why a safety net can save the life of a circus performer.
-
Communication expert Dianna Booher believes that "Humor anchors key points" and "makes your message memorable."19 Discuss the role of humor in business presentations.
-
A rectangular core has fixed permeability r >> 1, a square cross section of dimensions a a, and has centerline dimensions around its perimeter of b and d. Coils 1 and 2, having turn numbers N 1 and...
-
Modigliani and Miller (MM) on the one hand and Gordon and Lintner (GL) on the other hand have expressed strong views regarding the effect of dividend policy on a firms cost of capital and value. a....
-
A 5.00-F capacitor is initially chmged to a potential of 16.0 V. It is then connected in series with a 3.75-mH inductor. (a) What is the total energy stored in this circuit? (b) What is the maximum...
-
Establishing a capital structure for a firm is not simple. Although financial theory guides the process, there is no easy formula to determine a target debt-to-equity ratio. However, there are key...
-
In an economy described by the Specific Factors Model, the production possibility frontier will be a. convex to the origin. b. parabolic with one root. c. linear. d. collapsed to a point. e. concave...
-
How would you prepare the following compounds starting with an appropriate carboxylic acid and any other reagents needed? (Reddish brown =Br.) (a) (b)
-
Electrostatic potential maps of a typical amide (acetamide) and an acyl azide (acetyl azide) are shown. Which of the two do you think is more reactive in nucleophilic acyl substitution reactions?...
-
Charlotte maintains a provision for doubtful debts at 2 1/2% of her trade receivables at the end of each financial year. a. Explain the meaning of each of the following terms: i. Irrecoverable debt...
-
The following information about the payroll for the week ended December 30 was obtained from the records of Saine Co.: Salaries: Sales salaries Deductions: $180,000 Income tax withheld $65,296...
-
You have just been hired as the chief executive officer (CEO) in a medium-sized organization. The organization is not suffering financially, but neither is it doing as well as it could do. This is...
-
The following is the selling price and cost information about three joint products: X Y Z Anticipated production 1 2 , 0 0 0 lbs . 8 , 0 0 0 lbs . 7 , 0 0 0 lbs . Selling price / lb . at split - off...
-
calculate the maximum bending compressive stress of the following section under NEGATIVE bending moment of 216KN.m. 216mm 416mm 316mm 115mm
-
Need assistance with the following forms: 1040 Schedule 1 Schedule 2 Schedule C Schedule SE Form 4562 Form 8995 Appendix B, CP B-3 Christian Everland (SS number 412-34-5670) is single and resides at...
-
Recognize the importance to a hotel of properly managing and controlling its non-room revenue.
-
Accounting policies and practices that are most important to the portrayal of the companys financial condition and results, and require managements most difficult, subjective, or complex judgments...
-
Write orbital diagrams (boxes with arrows in them) to represent the electron configurations of carbon before and after sp hybridization.
-
How would you distinguish among the compounds within each of the following sets using their NMR spectra? Explain carefully and explicitly what features of the NMR spectrum you would use. (a)...
-
Give the structure of each of the following compounds. (In some cases, more than one correct answer is possible.) (a) A six-carbon alkene whose proton NMR spectrum consists of one singlet (b) A...
-
Give the structure of each of the following compounds. (In some cases, more than one correct answer is possible.) (a) A six-carbon alkene whose proton NMR spectrum consists of one singlet (b) A...
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


