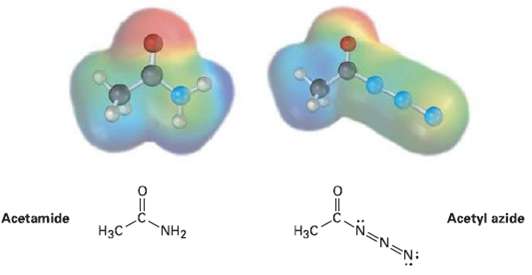
Electrostatic potential maps of a typical amide (acetamide) and an acyl azide (acetyl azide) are shown. Which
Question:
Electrostatic potential maps of a typical amide (acetamide) and an acyl azide (acetyl azide) are shown. Which of the two do you think is more reactive in nucleophilic acyl substitution reactions? Explain.
Transcribed Image Text:
Acetamide Acetyl azide NH2 Нзс Нас
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
According to the electrostatic potential maps the carbonyl carbon of ac...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which store do you think is more expensive-physical or online? A recent survey (USA Today, December 10, 2012, p. 1B) found that 46% of people aged 20 to 40 thought that physical stores were more...
-
Which of the alkyl halides is more reactive in an E2 reaction? a. CH3CH2CH2Br or b. c. d. CH,CH2CHCH3 Cl or CH cHy CH3CHCH2CHCH or CH3CH2CH2CCH3 Br Br CH, CH CH3 CH3
-
Which company and business model do you think is most likely to dominate the Internet and why ?
-
Give the approximate temperature at which creep deformation becomes an important consideration for each of the following metals: nickel, copper, iron, tungsten, lead, and aluminum.
-
What are detractors saying about PowerPoint, and why are they condemning it? Can you present a counterargument?
-
In Problem 8.28, the linear approximation suggested in the statement of the problem leads to flux density of 0.666 T in the central leg. Using this value of B and the magnetization curve for silicon...
-
Would it ever be rational for a firmto borrowmoney in order to pay cash dividends? Explain. AppendixLO1
-
In a monopolistically competitive market, the government applies a specific tax of $ 1 per unit of output. What happens to the profit of a typical firm in this market? Does the number of firms in the...
-
How were the Coca-Cola cash flows recently affected by exchange rate movements according to its annual report? (Note- cash flows would be affected by transaction and economic exposure). Review the...
-
Shown below are the summarized final accounts of Martel plc for the last two financial years: Summarised statement of comprehensive income for the year ending 31 December Additional information: 1...
-
The following structure represents a tetrahedral alkoxide-ion intermediate formed by addition of a nucleophile to a carboxylic acid derivative. Identify the nucleophile, the leaving group, the...
-
Give IUPAC names for the following compounds: o (c) CHH2H2 CH2CH3 (a) (b) CH3CH2CHCHI NH2 (d) (e) (f) CH3CHCH,NHCH3 CH-CH-C H CH Br (h) (g) SCH(CH3)2
-
The financial statements of Noble Employment Services, Inc., reported the following accounts (adapted, with dollar amounts in thousands except for par value): Net income has already been closed to...
-
Units processed during September for material and conversion. Ask an instructor lock lock lock A 3 A copy Determine the cost per equivalent unit for material and conversion cost combined. copy...
-
12% of all college students volunteer their time. Is the percentage of college students who are volunteers different for students receiving financial aid? Of the 338 randomly selected students who...
-
Mervon Company has two operating departments: mixing and bottling. Mixing has 3 3 0 employees and Bottling has 2 2 0 employees. Indirect factory costs include administrative costs of $ 1 8 2 , 0 0 0...
-
XP Ltd. is a manufacturing company with high stock requirements. Management are currently considering their stockholding policy. The following information is available for one stock item, material...
-
Process Costing: weighted average method Required: make a cost of production report in good form. Cost of Production Report-Weighted Average First Dept- Gem Company applies 100% of materials at the...
-
Jerry Dickson has been approached by the franchise sales representative of a major hotel chain. The sales representative is trying to interest Jerry in building one of the franchise brands...
-
A company has the following incomplete production budget data for the first quarter: In the previous December, ending inventory was 200 units, which was the minimum required, at 10% of projected...
-
Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp 3 hybridization.
-
Give the structure that corresponds to each of the following molecular formulas and NMR spectra: (a) C5H10; 1.5, s (b) C2H2F3I: 3.56 (q, J = 10 Hz) (c) C6H14O: 0.91 (6H, d, 7 = 7 Hz); 1.17(6H,...
-
A compound A reacts with H2 over Pd/C to give methylcyclohexane. A colleague, A1 Keen, has deduced that the compound must be either 1-methylcyclohexene or 3-methylcyelohexene. You have been called in...
-
A compound A reacts with H2 over Pd/C to give methylcyclohexane. A colleague, A1 Keen, has deduced that the compound must be either 1-methylcyclohexene or 3-methylcyelohexene. You have been called in...
-
44. Dryer Companys policy is to keep 25% of the next month's sales in ending inventory. If Dryer meets its ending inventory policy at the end of April and sales are expected to be 24,000 units in May...
-
What general conclusions can you draw about your companys liquidity, solvency and productivity based on your ratio calculations. Working Capital 2017 = $9,994 M 2016 = $10,673 M Current Ratio 2017 =...
-
Tami Tyler opened Tami's Creations, Incorporated, a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain...

Study smarter with the SolutionInn App


