Give IUPAC names for the following compounds: o (c) CHH2H2 CH2CH3 (a) (b) CH3CH2CHCHI NH2
Question:
Give IUPAC names for the following compounds:
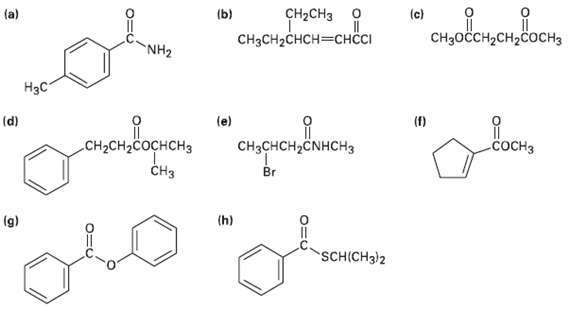
Transcribed Image Text:
oн (c) CHзоссH2сH2соснз CH2CH3 о (a) (b) CH3CH2CHCH—снссI NH2 Нзс (d) (e) (f) CH3CHCH,ČNHCH3 CH-CH-Cоснснз сосHз CHз Br (h) (g) SCH(CH3)2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (22 reviews)
a d H3C pMethylbenzamide g NH Isopropyl 3phenylpropanoate b CH3...View the full answer

Answered By

Sandip Nandnawar
I am a B.E (Information technology) from GECA and also have an M.C.M from The University of RTMNU, MH.
I worked as a software developer (Programmer and TL). Also working as an expert for the last 6 years and deal with complex assessment and projects. I have a team and lead a team of experts and conducted primary and secondary research. I am a senior software engg and senior expert and deal with all types of CSE and IT and other IT-related assessments and projects and homework.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Give IUPAC names for the following compounds: CH CH3CH2CH=CCH2CH3 (b) (a) CH CHCH-H CHCH2CH2CHCHCHCH2CH3 CH CH (d) (c) CHCHCHCHC CH
-
Give IUPAC names for the followingcompounds: H CHCH2CCH H CH CHH2CH2CH (b) (e) CHH2H2C CH (a) H-H H CH3CH2CHCH2CH,CHCH3 (e) CH H CHCH2CH2CHCH2CH CH CH-H>H3 (d)
-
Give IUPAC names for the following alkenes: (a) (b) (c) (d) (e) (f) OH CI
-
Compare sand, die, investment, lost foam, and continuous casting techniques.
-
"Communicate-don't decorate." This principle is one of 20 rules that graphic designer and educator Timothy Samara discusses in his book Design Elements: A Graphic Style Manual. How could you apply...
-
1. How is the number of customers who arrive on the lot on a Saturday morning distributed? 2. Suppose Ms. McNeil currently uses five salespeople on her lot on Saturday mornings. Using the probability...
-
The cost of retained earnings is less than the cost of new outside equity capital. Consequently, it is totally irrational for a firm to sell a new issue of stock and to pay cash dividends during the...
-
Given the circuit shown in Fig. 19-36, use the words increase, decrease, or stays the same to complete the following statement: (a) If R7 increases, the potential difference between A and E____....
-
What is hedging? Explain..I am not satisfy give downvote
-
1. Write a short description of the four types of housing generally available for Seyed. 2. List several sources of information applicable to any real estate purchase that might be helpful to Seyed...
-
Electrostatic potential maps of a typical amide (acetamide) and an acyl azide (acetyl azide) are shown. Which of the two do you think is more reactive in nucleophilic acyl substitution reactions?...
-
Draw structures corresponding to the following names: (a) p-Bromophenylacetamide (b) m-Benzoylbenzamide (c) 2, 2-Dimcthylhcxanamidc (d) Cyclohexyl cyclohexanecarboxylale (e) Ethyl...
-
Holtzman Clothierss stock currently sells for $38.00 a share. It just paid a dividend of $2.00 a share (i.e., D 0 = $2 00). The dividend is expected to grow at a constant rate of 5% a year. What...
-
Please help! I'm stuck 1) What purpose would your computer system serve? Business or personal or both? 2) Is this laptop/portable or desktop with monitor attached or all-in-one desktop? 3) What would...
-
The airline industry is severely hit by the COVID-19. Rows 6 to 85 show the daily closing prices of three stocks (i.e.,Qantas Airways Limited (QAN.AX), Singapore Airlines Limited (C6L.SI), and Cathay...
-
Using C+ Write a program to let users input two integers. If the first number is greater than the second number, print "The first number is larger". If the second number is greater than the first...
-
7. The normal model Show that if the risk-neutral distribution of ST is given by ST | S ~N (F, (T-t)), where F = F(t, T)istheforwardprice, thenthepriceofa K-strike straddle is approxim- ated by Z(t,...
-
25 cm 75 cm Water Parabola 2. The wheel-well of a custom truck-mounted water tank has a semi- parabolic shape as shown (assume point A corresponds to the peak). It's width is projected 150 cm into...
-
Jerielle Pelley is the front office manager at the 125-room Best Stay Inn. Her general manager has asked her to prepare a Net ADR Yield report for the hotels prior month room sales. The manager has...
-
Interview managers at three companies in your area about their use of ERP. How have their experiences been similar? What accounts for the similarities and differences?
-
Write orbital diagrams (boxes with arrows in them) to represent the electron configurationswithout hybridizationfor all the atoms in SF 2 . Circle the electrons involved in bonding. Draw a...
-
How many absorptions should be observed in the 13C NMR spectrum of each of the following compounds? (Assume that the chair interconversion is rapid.) CH3 H,C
-
Explain how the proton NMR spectra of the compounds within each of the following sets would differ, if at all. (CH)2CHC and (CH CDCI
-
Although this chapter has discussed only nuclei that have spin 1/2, several common nuclei such as 14N and deuterium (2H, or D) have a spin of 1. This means that the spin has three equally probable...
-
Ray Company provided the following excerpts from its Production Department's flexible budget performance report. Required: Complete the Production Department's Flexible Budget Performance Report....
-
Problem 1 5 - 5 ( Algo ) Lessee; operating lease; advance payment; leasehold improvement [ L 0 1 5 - 4 ] On January 1 , 2 0 2 4 , Winn Heat Transfer leased office space under a three - year operating...
-
Zafra and Stephanie formed an equal profit- sharing O&S Partnership during the current year, with Zafra contributing $100,000 in cash and Stephanie contributing land (basis of $60,000, fair market...

Study smarter with the SolutionInn App


