Question: A copper-nickel diffusion couple similar to that shown in Figure 5.1a is fashioned. After a 700-h heat treatment at 1100?C (1373 K) the concentration of
A copper-nickel diffusion couple similar to that shown in Figure 5.1a is fashioned. After a 700-h heat treatment at 1100?C (1373 K) the concentration of Cu is 2.5 wt% at the 3.0-mm position within the nickel. At what temperature must the diffusion couple need to be heated to produce this same concentration (i.e., 2.5 wt% Cu) at a 2.0-mm position after 700 h? The preexponential and activation energy for the diffusion of Cu in Ni are given in Table 5.2.
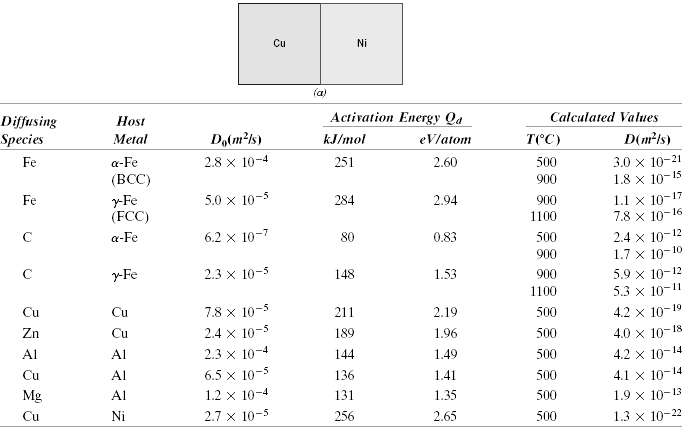
Cu Ni (a) Activation Energy Qa Calculated Values Host Diffusing Species Dalm?1s) D(mls) ) Metal kJ/mol eVlatom 3.0 x 10-21 1.8 x 10-15 2.8 x 10-4 Fe a-Fe 251 2.60 500 (BCC) 900 1.1 x 10-17 7.8 x 10-16 y-Fe (FCC) 5.0 x 10-5 284 2.94 900 Fe 1100 2.4 x 10-12 1.7 x 10-10 6.2 x 10-7 a-Fe 80 0.83 500 900 5.9 x 10-12 5.3 x 10-11 2.3 x 10-3 r-Fe 148 1.53 900 1100 4.2 x 10-19 Cu Cu 7.8 x 10-5 211 2.19 500 18 2.4 x 10-5 4.0 x 10- Zn Cu 189 1.96 500 2.3 x 10-4 6.5 x 10-5 Al 144 500 Al 1.49 4.2 x 10-14 4.1 x 10-14 Cu Al 136 1.41 500 10-4 Mg Al 1.2 131 1.35 500 1.9 x 10-13 2.7 x 10-5 1.3 x 10-22 Cu Ni 256 2.65 500
Step by Step Solution
3.33 Rating (162 Votes )
There are 3 Steps involved in it
In order to determine the temperature to which the diffusio... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (153).docx
120 KBs Word File


