What product is obtained when ethylamine reacts with excess methyl iodide in a basic solution of potassium
Question:
What product is obtained when ethylamine reacts with excess methyl iodide in a basic solution of potassium carbonate?
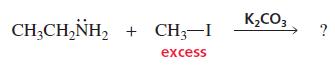
Transcribed Image Text:
K,CO3 CH;CH,NH, + CH3-I excess
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)

Answered By

Shubham Belsare
I'm currently pursuing Post graduation in organic chemistry and I'm passionately driven to teach Organic Chemistry to the students who finds hard time learning the subject. Apart from my regular college, I'm also a part-time subject expert on Chegg and I very much like and enjoy clearing the doubts of students which also increase my knowledge toward the subject.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the major product(s) that are expected when each of the following amines is treated with excess methyl iodide and then heated in the presence of aqueous silver oxide. (a) (b) NH2 NH2
-
Only a substitution product is obtained when the following compound is treated with sodium methoxide: Explain why an elimination product is not obtained. CH3 Br CH3
-
What products would be obtained when acetophenone reacts under each of the following conditions? (a) (b) (c) (d) (e) (f) (g) Acetophenone HNO H2S04 CBH NHNH2, HA NaBH CH2OH (1) CBHMgBr (2) NHC...
-
(2) Use Figure 10.2 shows a multi-degree-of-freedom (MDOF) model of three connected masses. Find the following for the MDOF system in Figure 10.2: Find expressions for the kinetic energy (T) and...
-
Explain how the difference voltage in Figure 19-35 reduces noise from source flicker.
-
Express versus Implied Contracts. Suppose that a local businessperson, McDougal, is a good friend of Krunch, the owner of a local candy store. Every day on his lunch hour, McDougal goes into Krunchs...
-
Use the applet entitled Correlation by the Eye to explore the relationship between the pattern of data in a scatterplot and the corresponding correlation coefficient. LO9 a. Run the applet several...
-
1. Which inventory costing method generally results in less current taxes paid by the company? a. LIFO b. FIFO c. Moving Average d. All methods result in the same taxes paid 2. Which inventory...
-
Under the modified accrual basis of accounting, expenditures generally are not recognized until: Goods or services are ordered. They are paid in cash. They are approved by the legislative body . An...
-
Southeastern Airliness daily flight from Atlanta to Charlotte uses a Boeing 737, with all-coach seating for 120 people. In the past, the airline has period every seat at $140 for the one-way flight....
-
List the following species in order of decreasing nucleophilicity in an aqueous solution: CH;OH HO CH;CO CH3S
-
Most of the pK a values given throughout this text are values determined in water. How would the pK a values of the following classes of compounds change if they were deter-mined in a solvent less...
-
Given the following business rules, construct an ER diagram so each rule is captured for the database. Presume each rule is to be treated individually, so construct an ER diagram for each rule. a. A...
-
What work trait differences are similar in chart 1 and chart 2? Provide a comment for each of the 4 generations from each chart. Which work trait differences vary from those identified in chart 1 and...
-
Given the ALU design illustrated below, without changing the circuit design, please use the ALU to perform a logic NAND operation. Find out what the control signals should be (i.e. the values of...
-
Problem #5: Using the method of joints, determine the force in each member. State whether each member is in compression or tension. If the largest force each member can support is 4kN tension and 3kN...
-
Your cultural/social background and that of your family. What language, policies/structures and customs are relevant to your own culture? How do you think your own background impacts on people from...
-
In this second Case Assignment, the assignment is going to test your understanding of how successful teams operate efficiently through teamwork. Teamwork relies upon individuals to work together to...
-
Calculate the iterated integral. 1-y2 y sin x dz dy dx Jo Jo
-
Chloroplasts are illuminated until the levels of the Calvin cycle intermediates reach a steady state. The light is then turned off. How does the level of RuBP vary after this point?
-
Lysozyme is an antibacterial enzyme that hydrolyzes polysaccharides in bacterial cell walls. It also catalyzes the hydrolysis of a -1,4-linked hexasaccharide oligomer of N-acefylglucosamine into a...
-
Lysozyme is an antibacterial enzyme that hydrolyzes polysaccharides in bacterial cell walls. It also catalyzes the hydrolysis of a -1,4-linked hexasaccharide oligomer of N-acefylglucosamine into a...
-
Classify the following peptides as acidic, basic, or neutral. What is the net charge on each peptide at pH = 6? (a) Gly-Leu-Val (b) Leu-Trp-Lys-Gly-Lys (c) N-acetyl-Asp-Val-Ser-Arg-Arg (A-acetyl...
-
Docs Auto Body has budgeted the costs of the following repair time and parts activities for 2009: Doc's budgets 6,000 hours of repair time in 2009. A profit margin of $7 per labour hour will be added...
-
QUESTION 28 In a perpetual inventory system, the cost of inventory sold is: Debited to accounts receivable. Debited to cost of goods sold. O Not recorded at the time goods are sold. O Credited to...
-
The following financial statements and additional information are reported. IKIBAN INC. Comparative Balance Sheets June 30, 2019 and 2018 2019 2018 $105,709 69,500 66,800 4,700 246,700 127,eee...

Study smarter with the SolutionInn App


