List the following species in order of decreasing nucleophilicity in an aqueous solution: CH;OH HO CH;CO CH3S
Question:
List the following species in order of decreasing nucleophilicity in an aqueous solution:
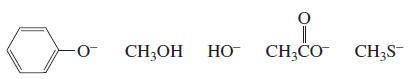
Transcribed Image Text:
CH;OH HO CH;CO CH3S
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
Nucleophilicity Nucleophilicity increases as the charge on the atom increases In aqueous solution fo...View the full answer

Answered By

Akshay Nagar
Experience:-
I have 2 years of experience of teaching Physics to class understudies as well as other plateforms.In my day to day classes I teach students about Numericals,Concepts,Data Collection,Exam preparation and a whole spectrum of Physical Science.
Qualifications:-
1. Bachelor of Science with Maths and Physics major (MDU,Rohtak, India)
2. Bachelor of Education (CBLU, Bhiwani,India)
3. Masters of physics (MDU, Rohtak, India)
4. Teach English certified teacher (Arizona state university,Tempe, Arizona)
5. Specialization in Electronic Music Production (Berklee College Of Music, Boston,Massachusetts)
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
List the following species in order of decreasing basicity: a. b. CH,CHC CHCH NH2 CH,CH20 F
-
List the following compounds in order of decreasing reactivity in an E2 reaction: CH CD CH CH3 CD
-
List the following compounds in order of decreasing boiling point: OH NH2 HO
-
A PLC is used to count the number of cans traveling by on a conveyor belt in a fish canning factory. An optical proximity switch detects the passage of each can, sending a discrete (on/off) signal to...
-
A measurement with a signal-to-noise ratio of 100/1 can be thought of as a signal, S, with 1% uncertainty, e. That is, the measurement is S e = 100 1. (a) Use the rules for propagation of...
-
Draw and explain the life cycle model of leadership. Would this model be useful to you as a manager? Why or why not? LO6
-
Calculate r2 for the least squares line in Exercise 11.21 LO9 (p. 625).
-
Olivia Company began 2013 with a Retained Earnings account balance of $180,000. During 2013, the following 8 events occurred and were properly recorded by the company: 1. Bonds payable with a face...
-
Before you Begin: Additional Information to solve the question: 1 . Increase in Sales Percentage will be assumed to apply only to COGS on the Income Statement. 2 . Depreciation and Amortization,...
-
The Western Jeans Company purchases denim from Cumberland Textile Mills. The Western Jeans Company uses 35,000 yards of denim per year to make jeans. The cost of ordering denim from the textile...
-
a. Which is a stronger base, RO - or RS - ? b. Which is a better nucleophile in an aqueous solution?
-
What product is obtained when ethylamine reacts with excess methyl iodide in a basic solution of potassium carbonate? K,CO3 CH;CH,NH, + CH3-I excess
-
(a) A 1 megabit memory is organized in a square with each memory cell being individually addressed. Determine the number of input address lines required for the row and column decoders. (b) If the 1...
-
Think back to a time you experienced a communication breakdown in a personal or social setting (something you're comfortable discussing with the class in a public forum). 1. Did you figure out why...
-
Imagine you are visiting your aunt, who is a patient in a hospital in a nearby city. While you are sitting at her bedside, you hear a lot of noise at the nurses' station, as if they are having a...
-
Using Houseplan #5 on page 4 of the Measurement supplement(below), determine the cost of pouring the 9 inch thick concreteslab for this home, assuming that the porch will also be on thefoundation....
-
Recall from lecture that Flip-Flap Railway is an old roller coaster that was built in a circle. It has a diameter of 25 ft and riders entered the ride at a speed of 45 mph. At the top of the loop,...
-
Small Fry Design, founded in 1997, is a toy and accessories company that designs and imports products for children. The company's line of merchandise includes teddy bears, musical toys, rattles and...
-
Sketch the region whose area is given by the integral and evaluate the integral. 37/4 (2 r dr de
-
For the following arrangements, discuss whether they are 'in substance' lease transactions, and thus fall under the ambit of IAS 17.
-
(a) In most peptides, the amide bonds have the Z conformation; explain why. (b) One particular amino acid residue in the PepC position adopts the E conformation in some cases. Which amino acid...
-
When N - acetyl- L -aspartic acid is treated with acetic anhydride, an optically active compound A, C6H7NO4, is formed" Tleaffient of A with the amino acid L -alanine yields two separable, isomeric...
-
When N - acetyl- L -aspartic acid is treated with acetic anhydride, an optically active compound A, C6H7NO4, is formed" Tleaffient of A with the amino acid L -alanine yields two separable, isomeric...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


