Draw the major product(s) that are expected when each of the following amines is treated with excess
Question:
(a)
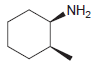
(b)
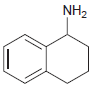
Transcribed Image Text:
NH2 NH2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (16 reviews)

Answered By

Vincent Omondi
I am an extremely self-motivated person who firmly believes in his abilities. With high sensitivity to task and operating parameters, deadlines and keen on instructions, I deliver the best quality work for my clients. I handle tasks ranging from assignments to projects.
4.90+
109+ Reviews
314+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the products that are expected when each of the following alkenes is treated with a peroxy acid (such as MCPBA) followed by aqueous acid: a. b. c. d. e. f.
-
Predict the products that are expected when each of the following alkenes is treated with ozone followed by DMS: a. b. c. d. e. f.
-
What product(s) are expected when each of the following compounds reacts with one equivalent of NBS in CC14 in the presence of light and peroxides? Explain your answers. (a) cyclohexene (b)...
-
In Fig. P2.72 gate AB is circular. Find the moment of the hydrostatic force on this gate about axis A. Neglect atmospheric pressure.
-
Find the sum of the finite geometric sequence. 1. 2. 3. 4. -1 2i i=1 31 i=1
-
1. Create both the written plan and the educational material to help African American women age 65+ control high blood pressure, take the special circumstances into consideration for the plan. 2. For...
-
P 16-11 Partnership income allocationMultiple years Har, Ion, and Jer formed a partnership on January 1, 2016, with each partner contributing $20,000 cash. Although the partnership agreement provided...
-
Return on investment ( ROI) is computed in the fol-lowing manner: ROI is equal to turnover multiplied by earnings as a percent of sales. Turnover is sales divided by total investment. Total...
-
Question 7 4 pts Following is a table for the present value of $1 at compound interest: Following is a table for the present value of an annuity of $1 at compound interest: Using the tables provided,...
-
Calculate the final pressure of a sample of water vapour that expands reversibly and adiabatically from 87.3 Torr and 500 dm3 to a final volume of 3.0 dm3. Take Y = 1.3
-
Consider the structure of the azo dye called alizarine yellow R (below). Show the reagents you would use to prepare this compound via an azo coupling process. .N. N' O2N
-
If Davis adds a line of apparel or shoes, should he try to sell his line to other retail clothing shops in Calgary or elsewhere in Canada?
-
Use a computer algebra system to evaluate the integral where C is represented by r(t). So C F. dr
-
3. The Balance Sheet of International Operators Ltd. as at 31.03.2021 disclose the following position: PARTICULARS SHARE CAPITAL RESERVES AND SURPLUS SECURED LOANS UNSECURED LOANS CURRENT LIABILITY...
-
A uniformly charged ring of radius a. (a) The field at P on the x axis due to an element of charge dq. (b) The perpendicular component of the field at P due to segment 1 is canceled by the...
-
At what rate would $1,000 have to be invested to grow to $4,046 in 10 years?
-
Add F1 and F2 using graphical method, (triangle or parallelogram) Determine: 1 Magnitude,2. Direction measured CCW from positive axis, im now to America need help. CoursHeroTranscribedText 20 F-SON...
-
What is Monetary Policy? What is Monetary Base or High Powered Money? How commercial Banks create money Supply? Hint: By giving loans through creating checking account What is deposit multiplier?...
-
The figure shows the cost of mailing a first-class letter, f(x), as a function of its weight, x, in ounces, for a recent year. Use the graph to solve Exercises 111114. Find f(3). What does this mean...
-
What are the 5 Cs of marketing channel structure?
-
Predict which compound in each pair has the higher boiling point. Explain your prediction. (a) CH3CH2OCH3 or CH3CH1OH2CH3 (b) CH3CH2CH2CH3 or CH3CH2CH2CH2CH3 (c) CH3CH2CH2CH2CH3 or (CH3)2CHCH2CH3 (d)...
-
Circle the functional groups in the following structures. State to which class (or classes) of compounds the structure belongs. (a) (b) (c) (d) (e) (f) (g) (h) (i) COOH N--H CH2OCH3 CN NH CH3 CHCOOCH...
-
Dimethyl sulfoxide (DMSO) has been used as an anti-inflammatory rub for race horses. DMSO and acetone appear to have similar structures, but the C=O carbon atom in acetone is planar, while the S=O...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...

Study smarter with the SolutionInn App


