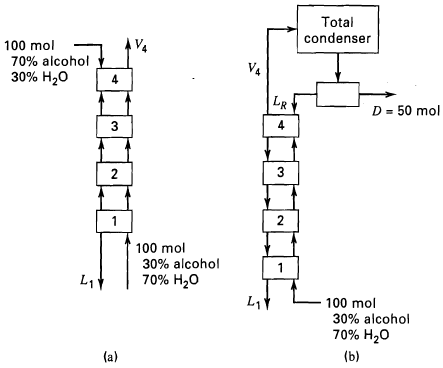
Question: (a) For the cascade shown in Figure a, calculate the compositions of streams V4 and L1. Assume atmospheric pressure, saturated liquid and vapor feeds, and
(a) For the cascade shown in Figure a, calculate the compositions of streams V4 and L1. Assume atmospheric pressure, saturated liquid and vapor feeds, and the vapor-liquid equilibrium data given below. Compositions are in mole percent.
(b) Given the feed compositions in cascade (a), how many equilibrium stages are required to produce a V4 containing 85 mol% alcohol?
(c) For the cascade configuration shown in Figure b, with D = 50 mol, what are the compositions of D and L1?
(d) For the configuration of cascade (b), how many equilibrium stages are required to produce a D of 50 mol%alcohol?

EQUILIBRIUM DATA, MOLE-FRACTION ALCOHOL 0.3 0.5 0.9 0.1 0.5 0.68 0.7 0.82 0.2 0.94
Step by Step Solution
3.36 Rating (168 Votes )
There are 3 Steps involved in it
Subject Separation of ethyl alcohol and water at 1 atm with two countercurrent cascades Given One cascade a with given liquid feed to top stage and gi... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (252).docx
120 KBs Word File


