Question: A fuel gas produced by gasifying coal is to be burned with 20% excess air. The gas contains 50.0 mole% nitrogen and the balance carbon
A fuel gas produced by gasifying coal is to be burned with 20% excess air. The gas contains 50.0 mole% nitrogen and the balance carbon monoxide and hydrogen. A sample of the gas is passed through an infrared spectrometer, which registers a signal R that depends on the mole fraction of carbon monoxide in the sample and a reading R 38.3 is recorded. Analyzer calibration data are as follows: A power law (x = aRb) should be suitable for fitting the calibration data. Derive the equation relating x and R (use a graphical method), and then calculate the molar flow rate of air required for a fuel feed rate of 175kmol/h, assuming that CO and H2 are oxidized but N2 is not.
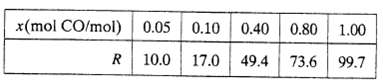
x(mol CO/mol) 0.05 0.10 0.40 17.0 49.4 R 10.0 0.80 1.00 73.6 73.6 99.7
Step by Step Solution
3.50 Rating (170 Votes )
There are 3 Steps involved in it
co 10 CO 175 kmolh 0500 kmol Nkmol x kmol COmol 0500x kmol Hkmol 20 exces... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (164).pdf
180 KBs PDF File
13-E-C-E-C-P (164).docx
120 KBs Word File


