The wastewater treatment plant at the Ossabaw Paper Company paper mill generates about 24 tonnes of sludge
Question:
The wastewater treatment plant at the Ossabaw Paper Company paper mill generates about 24 tonnes of sludge per day. The consistency of the sludge is 35%, meaning that the sludge contains 35 wt% solids and the balance liquids. The mill currently spends $40/tonne to dispose of the sludge in a landfill. The plant environmental engineer has determined that if the sludge consistency could be increased to 75%, the sludge could be incinerated (burned) to generate useful energy and to eliminate the environmental problems associated with landfill disposal.
A flowchart for a preliminary design of the proposed sludge-treatment process follows. For simplicity, we will assume that the liquid in the sludge is just water.
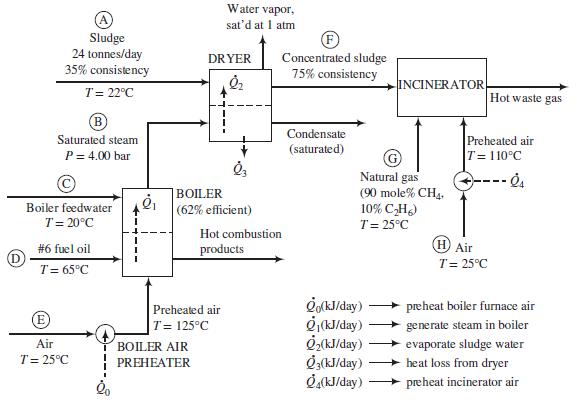
Process description: The sludge from the wastewater treatment plant (Stream Ⓐ ) passes through a dryer where a portion of the water in the sludge is vaporized. The heat required for the vaporization comes from condensing saturated steamat 4.00 bar (Stream Ⓑ). The steam fed to the dryer is produced in the plant’s oil-fired boiler from feedwater at 20°C(Stream Ⓒ). The heat required to produce the steamis transferred from the boiler furnace, where fuel oil (Stream Ⓓ) is burned with 25% excess air (Stream Ⓔ). The concentrated sludge coming from the dryer (Stream Ⓕ), which has a consistency of 75%, is fed to an incinerator. The heating value of the sludge is insufficient to keep the incinerator temperature high enough for complete combustion, so natural gas (Stream Ⓖ) is used as a supplementary fuel. A stream of outside air at 25°C (Stream Ⓗ) is heated to 110°C and fed to the incinerator along with the concentrated sludge and natural gas. The waste gas from the incinerator is discharged to the atmosphere.
Fuel oil: The oil is a low-sulfur No. 6 fuel oil. Its ultimate (elemental) analysis on a weight basis is 87% C, 10% H, 0.84% S, and the balance oxygen, nitrogen, and nonvolatile ash. The higher heating value of the oil is 3:75 × 104 kJ/kg and the heat capacity is Cp = 1:8 kJ/(kg ∙ °C).
Boiler: The boiler has an efficiency of 62%, meaning that 62% of the heating value of the fuel oil burned is used to produce saturated steam at 4.00 bar from boiler feedwater at 20°C. Fuel oil at 65°C and dry air at 125°C are fed to the boiler furnace. The air feed rate is 25% in excess of the amount theoretically required for complete consumption of the fuel.
Sludge: The sludge from the wastewater treatment plant contains 35% w/w solids (S) and the balance liquids (which for the purposes of this problem may be treated as only water) and enters the dryer at 22°C. The sludge includes a number of volatile organic species, some of which may be toxic, and has a terrible odor. The heat capacity of the solids is approximately constant at 2.5 kJ/(kg ∙ °C).
Dryer: The dryer has an efficiency of 55%, meaning that the heat transferred to the sludge, Q̇2, is 55% of the total heat lost by the condensing steam, and the remainder, Q̇3, is lost to the surroundings. The dryer operates at 1 atm, and the water vapor and concentrated sludge emerge at the corresponding saturation temperature. The steam condensate leaves the dryer as a liquid saturated at 4.00 bar.
Incinerator: The concentrated sludge has a heating value of 19,000 kJ/kg dry solids. For a feed sludge of 75%consistency, the incinerator requires 195 SC Mnatural gas/tonne wet sludge [1 SCM = 1m3(STP)]. The theoretical air requirement for the sludge is 2.5 SCMair/10,000 kJ of heating value. Air is fed in 100% excess of the amount theoretically required to burn the sludge and the natural gas.
(a) Use material and energy balances to calculate the mass flow rates (tonnes/day) of Streams
Ⓑ, Ⓒ, Ⓓ, Ⓔ, Ⓕ, Ⓖ, and Ⓗ, and heat flows Q̇0, Q̇1, . . . , Q̇4(kJ/day). Take the molecular weight of air to be 29.0. (Caution: Before you start doing lengthy and unnecessary energy balance calculations on the boiler furnace, remember the given furnace efficiency.)
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





