(a) How much ethylene glycol must be added to 47.3 kg of terephthalic acid to produce a...
Question:
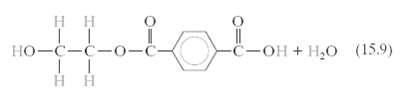
(a) How much ethylene glycol must be added to 47.3 kg of terephthalic acid to produce a linear chain structure of poly(ethylene terephthalate) according to Equation 15.9?
(b) What is the mass of the resultingpolymer?
Transcribed Image Text:
нн (15.9) но-с-с-о-с -С-он + Н, Нн
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
a This problem asks that we determine how much ethylene glycol must be added to 473 kg of terephthal...View the full answer

Answered By

Amit Kumar
I am a student at IIT Kanpur , which is one of the prestigious colleges in INDIA.
Cleared JEE Advance in 2017.I am a flexible teacher because I understand that all students learn in different ways and at different paces. When teaching, I make sure that every student has a grasp of the subject before moving on.
I will help student to get the basic understanding clear. I believe friendly behavior with student can help both the student and the teacher.
I love science and my students do the same.
4.90+
44+ Reviews
166+ Question Solved
Related Book For 

Fundamentals of Materials Science and Engineering An Integrated Approach
ISBN: 978-1118061602
4th Edition
Authors: David G. Rethwisch
Question Posted:
Students also viewed these Materials Science Engineering questions
-
(a) How much ethylene glycol must be added to 20.0 kg of dimethyl terephthalate to produce a linear chain structure of poly(ethylene terephthalate) according to Equations 15.9? (b) What is the mass...
-
What mass of steam at 100oC must be added to 1.00 kg of ice at 0oC to yield liquid water at 20oC?
-
What mass of sodium formate must be added to 500.0 mL of 1.00 M formic acid to produce a buffer solution that has a pH of 3.50?
-
Rafael Espinal's leadership approach is best categorized as what type of leadership? spiritual adaptive authentic servant
-
Ratio analysis can provide both internal and external users with a tremendous amount of information about firm performance. Select a publicly traded firm. Conduct a ratio analysis of the firm and...
-
The following control procedures are used at Newton Company for payroll. 1. The human resources department requires a background check for each employee. 2. Wage employees arrive to work when they...
-
E19-6 Compute the 20X5 cost of goods manufactured and cost of goods sold for Strike Marine Company using the following amounts: Materials inventory Work in process inventory...... Finished goods...
-
1. Using the purchase process (i.e., pre-purchase, purchase, and post-purchase), analyze the customer information provided by the owner and employees of Scholfield Honda. 2. Chapter 2 identifies...
-
Given the following balances, what is the most appropriate level of planning materiality for the 30 June 20X3 audit of your client?(all amounts are in $'000) Sales: 1310 (20X3); 1179 (20X2); 1048...
-
a. How much cash does Patterson have on hand relative to its total assets? b. What proportion of Patterson's assets has the firm financed using short-term debt? Long-term debt? c. What percent of...
-
Cite the primary differences between addition and condensation polymerization techniques.
-
Nylon 6, 6 may be formed by means of a condensation polymerization reaction in which hexamethylene diamine [NH2(CH2)6NH2] and adipic acid react with one another with the formation of water as a...
-
Is the change in the cash balance important to financial reporting and managerial analysis? Explain.
-
Consider the following account balances (in thousands) for the Shaker Corporation In the Dec 31.2021 Cash $200,000 and Capital $2,000,000 and Retained earnings $1,500,000 The balances of raw...
-
Unless otherwise stated, assume gravitational acceleration g = 9.81 m/s and the density of water to be 1000 kg/m. Unless otherwise stated, give all numerical answers to 3 significant figures, such as...
-
The purpose of this installment is to classify stock, bond, and mutual fund investments, explore tools for their evaluation and select these securities based on your investment philosophy and goals....
-
Jackson County Senior Services is a nonprofit organization devoted to providing essential services to seniors who live in their own homes within the Jackson County area. Three services are provided...
-
Caldwell (2003) explores differences between the roles of leaders and managers. "Leaders...envision, initiate, or sponsor strategic change of a far-reaching or transformational nature. In contrast,...
-
Why are work in process inventories sometimes omitted in preparing the manufacturing costs budget? LO.1
-
Heineken N.V., a global brewer based in the Netherlands, reports the following balance sheet accounts for the year ended December 31, 2016 (euros in millions). Prepare the balance sheet for this...
-
A balloon is filled with helium, and its volume is 2.2 L at 298 k. The balloon is then dunked into a thermos bottle containing liquid nitrogen. When the helium in the balloon has cooled to the...
-
What features can be incorporated into the gating system to aid in trapping dross and loose mold material that is flowing with the molten metal?
-
What features of the metal being cast tend to influence whether the gating system is designed to minimize turbulence and reduce dross, or promote rapid filling to minimize temperature loss?
-
What are the three stages of contraction or shrinkage as a liquid is converted into a finished casting?
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


