A problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes
Question:
A problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:
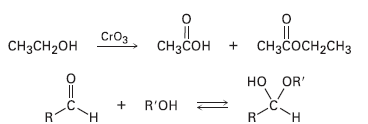
Transcribed Image Text:
|| CHзCон + снзҫосн-сHз CrOз CH3CH2OH но OR' + R'OH .C. н н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
An alcohol adds to an aldehyde by a mechanism that we will study in ...View the full answer

Answered By

Joan Gakii
I'm a meticulous professional writer with over five years writing experience. My skill set includes
- Digital Content,
- Interpersonal Communication,
- Web Content and academic Writing,
- Proofreading,
- Editing,
- Project Management, and
- Public Relations.
5.00+
7+ Reviews
12+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a mechanism to account for the formation of 3, 5-dimethylpyrazole from hydrazine and 2, 4-pcntancdionc. Look carefully to see what has happened to each carbonyl carbon in going from starting...
-
Propose a mechanism to account for the formation of Bakelite from acid-catalyzed polymerization of phenol and formaldehyde.
-
Overoxidation of primary alcohols to carboxylic acids is caused by the water present in the usual aqueous acidic Cr(VI) reagents. The water adds to the initial aldehyde product to form a hydrate,...
-
Bollobs and Chung proposed a hybrid model that combines a 2-ring on \(V\) vertices ( \(V\) is even), plus a random matching. A matching is a graph in which every vertex has degree 1. To generate a...
-
Which of the three basic philosophies of social responsibility would you find most appealing as the chief executive of a large corporation? Explain.
-
Hong Kong Stores accepts both its own and national credit cards. During the year, the following selected summary transactions occurred. Jan. 15 Made Hong Kong credit card sales totaling HK$18,000....
-
E 7-8 Midyear purchase of subsidiarys bonds Sanur Corporation is a 90 percent subsidiary of Pare Corporation. On January 1, 2016, Sanur issued $1,000,000 par, 10 percent 5-year bonds with an...
-
Briefly describe some of the similarities and differences between GAAP and IFRS with respect to the accounting for inventories.
-
UESTIC SPDR S&P Dividend (SDY) is an ETF that invests in highly profitable U.S. dividend stocks. Listed below are returns for SDY: SDY -6.55 % 11.61 % 30.07% 2013 2014 2015 2016 2017 -0.73 % 13.80 %...
-
There is a lottery with n coupons and n people take part in it. Each person picks exactly one coupon. Coupons are numbered consecutively from 1 to n, n being the maximum ticket number. The winner of...
-
Propose a synthesis of bicyclohexylidene, starting from Cyclohexanone as the only source of carbon. Bicyclohexylidene
-
Identify the reagents a?f in the following scheme: .Br CH-
-
What effects has the tort system had on the business community?
-
Give your overall opinion . What do you think about neuromarketing? Is it usefull or is it a waste of time? Some people think this practice is "Orwellian", do you agree? Can marketers manage the...
-
Question 1. Let z= f(x,y), x = g (s, t). and ' y = h (s, t). with f, g & h all differentiable. (a) Set up an appropriate tree diagram for the of chain rule as done in this module's Use video lessons:...
-
ow do synergistic dynamics emerge within high-performance teams, and what role do diverse skill sets, complementary roles, and shared goals play in fostering collaborative innovation and collective...
-
(14%) Problem 3: The circuit shown contains a voltage source with emf & = 5.99 V, a resistor with resistance R = 135 k2, and a capacitor with capacitance C = 507 nF. When switch S is set to position...
-
1. What functions do all managers perform regularly? How do these functions apply to the three levels of management found in most organizations? 2. Identify and distinguish between the different...
-
Apply portfolio analysis to guide decisions in companies with multiple products and businesses AppendixLO1
-
QUESTION 9 HC-O-C-R R-C-O-CH HC-O-P-O-CH-CH-NH3* O || O a. Phosphatidic acid, Serine O b. Lysophosphatidic acid, Serine, Free FA O c. Lysophosphatidylserine, Free FA O d. 2 Free FAs, Serine, Glycerol...
-
Is there any scientific basis for the colloquial expression slower than molasses in January? Explain.
-
When (S)-2-bromopropanoic acid [(S)-CH3CHBrCO2H] reacts with concentrated sodium hydroxide, the product formed (after acidification) is (R)-2-hydroxypropanoic acid [(R)-CH3CHOHCO2H, commonly known as...
-
Using chair conformational structures (Section 4.11), show the nucleophilic substitution reaction that would take place when trans-1-bromo-4-tert-butylcyclohexane reacts with iodide ion. (Show the...
-
The phenomenon of configuration inversion in a chemical reaction was discovered in 1896 by Paul Walden (Section 6.6). Walden's proof of configuration inversion was based on the following cycle: (a)...
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered
-
You plan to invest $10,00 today in an investment account earning 5% interest. You then plan to invest an additional $1,000 into this account each year for the next twenty years. How much money will...

Study smarter with the SolutionInn App


