(a) Show the unshared electron pairs on the following anion, The S has a formal charge of...
Question:
(a) Show the unshared electron pairs on the following anion, The S has a formal charge of ? 1, and the formal charges of the other atoms are zero.
(b) Draw a resonance structure for this ion,
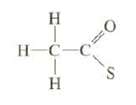
Transcribed Image Text:
Н н- H-C-C с Н 0 S
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 37% (8 reviews)
A doubly bonded oxygen needs 4 more electrons to satisfy ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Draw a resonance structure for each of the compounds below. a. b. c. N
-
The compound has a formal charge of (a) -1 on N (b) +2 on N (c) -1 on Al (d) +1 on Br (e) None of the above Br CH3 Br-Al-N-CH.CIH, Br CH3
-
Draw two resonance structures for diazomethane, CH2N2. Show formal charges. The skeletal structure of the molecule is C N N
-
(a) A dice is thrown n times where n is a sufficiently large enough number to provide a uniformly distributed set of results. What would be the conditions for the arrangement of the result set that...
-
Outline the three levels of customer-driven activities and provide two examples for each level.
-
Staal Enterprises is considering a change from its current capital structure. Staal currently has an all-equity capital structure and is considering a capital structure with 25 percent debt. There...
-
types of variables used in statistical analysis
-
1. Assume that the elasticity of demand for chewing tobacco is 0.70 and the elasticity of supply is 2.30. Suppose an antic hewing campaign decreases the demand for chewing tobacco by 18 percent. The...
-
Factors affecting the reliability of accounts receivable confirmation include the dollar value of accounts receivable being confirmed with the client
-
From the following T accounts of Brian?s Cleaning Service, (a) Foot and determine ending balances, (b) Prepare a trial balance in proper form for October 201X. Cash 111 Accounts Payable 211 Cleaning...
-
Predict the geometry at each atom, except hydrogen's, in these compounds: a) H --- 1 H b) H-c-c-c-
-
Show a Lewis structure for C 2 H 6 O in which both carbons are bonded to the oxygen. What is the geometry of this molecule at the oxygen? Show the direction of the dipole for the molecule,
-
Determine the horizontal and vertical components of reaction at the pins A, B, and C. A 10 kN/m - 4 m- Prob. F2-2 B 45 2 m C
-
solve for x 4 . 0 a 2 = 2 . 0 a x
-
BUSINESS SOLUTIONS Comparative Balance Sheets March 3 1 , 2 0 2 2 December 3 1 , 2 0 2 1 Assets Cash $ 8 4 , 7 8 7 $ 5 7 , 8 7 2 Accounts receivable 2 4 , 2 6 7 5 , 0 6 8 Inventory 6 1 4 0 Computer...
-
Solve:z-18=-103.
-
Complete the social penetration exercise and post your reactions in the discussion. PIRATION Purpose: 1. To help you understand the breadth and depth of self-disclosure. 2. To help you see the...
-
The implicit equation of x = sin ( t ) and y = 2 cos ( t ) is:
-
Assume that the following events occurred at a division of American Homes, Inc., a large homebuilder, for the current year. : Purchased $270 million in building material. Incurred construction labor...
-
According to a New York Times columnist, The estate tax affects a surprisingly small number of people. In 2003, . . . just 1.25 percent of all deaths resulted in taxable estates, with most of them...
-
Classify the following functions as one of the types of functions that we have discussed. (a) f(x) = 5x (c) h(x) = 1 + x 1- - (b) g(x) = x (d) u(t)=1t+5t
-
Draw the indicated number of resonance forms for each of the following species: (a) The methyl phosphate anion, CH3OPO32- (3) (b) The nitrate anion, NO3- (3) (c) The allyl cation, H2C = CH ? CH2+ (2)...
-
Nitric acid (HNO3) reacts with ammonia (NH3) to yield ammonium nitrate. Write the reaction, and identify the acid, the base, the conjugate acid product, and the conjugate base product.
-
The amino acid phenylalanine has pKa = 1.83, and tryptophan has pKa = 2.83. Which is the strongeracid? OH H3N H H3N H Tryptophan Phenylalanine (pka = 1.83) (pka = 2.83)
-
You have $55,000. You put 15% of your money in a stock with an expected return of 10%, $38,000 in a stock with an expected return of 18%, and the rest in a stock with an expected return of 22%. What...
-
Portfolio return and beta Personal Finance Problem Jamie Peters invested $ 1 1 3 , 0 0 0 to set up the following portfolio one year ago: a . Calculate the portfolio beta on the basis of the original...
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...

Study smarter with the SolutionInn App


