A solution contains two volatile liquids A and B. Complete the following table, in which the symbol
Question:
A solution contains two volatile liquids A and B. Complete the following table, in which the symbol → indicates attractive intermolecular forces.
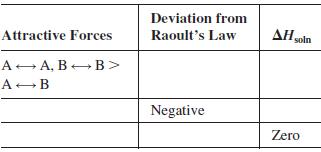
Transcribed Image Text:
Deviation from Attractive Forces Raoult's Law AH soln A A, B B> A-B Negative Zero
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (16 reviews)
The completed table is shown below The first row represents a Case 1 situat...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The volatile liquids A and B, for which P * A = 165 Torr and P * B = 85.1. Torr are confined to a piston and cylinder assembly. Initially, only the liquid phase is present. As the pressure is...
-
For a pure substance, the liquid and gaseous phases can only coexist for a single value of the pressure at a given temperature. Is this also the case for an ideal solution of two volatile liquids?
-
A mixture of liquids A and B exhibits ideal behavior. At 84C, the total vapor pressure of a solution containing 1.2 moles of A and 2.3 moles of B is 331 mmHg. Upon the addition of another mole of B...
-
Four of Wands, LLC generated $255,000 in sales during January 2022. Of this amount, 25% was for cash. The remaining 75% of sales were made on account. The February 2022 sales on account were...
-
Clark Paints: The production department has been investigating possible ways to trim total production costs. One possibility currently being examined is to make the paint cans instead of purchasing...
-
Moriarity and Holmes enter into an oral contract by which Moriarity promises to sell and Holmes promises to buy Blackacre for $10,000. Moriarity repudiates the contract by writing a letter to Holmes...
-
Identify whether actual laws were broken and where the line between unethical and illegal may have been crossed. (p. 249)
-
Famas Llamas has a weighted average cost of capital of 8.9 percent. The companys cost of equity is 12 percent and its cost of debt is 7.9 percent. The tax rate is 35 percent. What is Famas target...
-
Questions, Classroom Demonstration Exercises. Exercises, and Problems P3-2B. On April 1, 2023, Beth Orth opened Beth's Art Studio. The following transactions occurred in April: 2023 Apr. Beth Orth...
-
A circuit youre using discharges a 20 F capacitor through an unknown resistor. After charging the capacitor, you close a switch at t = 0 s and then monitor the resistor current with an ammeter. Your...
-
How does each of the following affect the solubility of an ionic compound? (a) Lattice energy, (b) Solvent (polar versus nonpolar), (c) Enthalpies of hydration of cation and anion
-
The concentration of commercially available concentrated sulfuric acid is 98.0 percent by mass, or 18 M. Calculate the density and the molality of the solution.
-
After evaluating Zero Companys manufacturing process, management decides to establish standards of 1.5 hours of direct labor per unit of product and $11 per hour for the labor rate. During October,...
-
From Hoffman, what are the symptoms of autism and ADHD? From the Mayo Clinic, what are the causes and risk factors for autism and ADHD? What are treatment options for these disorders? Hofmann, S. G....
-
Brooks, a participant in the Zappa retirement plan, has requested a second plan loan. His vested account balance is $80,000. Brooks borrowed $27,000 eight months ago and still owes $18,000 on that...
-
Suppose that Angelina and Brad own the only two professional photography stores in town. Each must choose between a low price and a high price for senior photo packages. The annual economic profit...
-
based on the article How Chili's Is Prepping for Tough Times, Starting With the Fries by Heather Haddon. What is corporate social responsibility, and what is one way that Chili's can better pursue...
-
QUESTION 2 (20 marks) CLO 5 a. Explain what the following ratios indicate to a firm: (i) Acid Test Ratio (ii) Return on Capital Employed (ROCE) (iii) Debtors Collection Period (iv) Working Capital (2...
-
Draw a beaker that shows the result of dissolving methanol, CH 3 O H , in water and a second beaker that shows the result of dissolving sodium phosphate in water. Indicate all species present in...
-
Give an example of transitory income. What effect does this income have on the marginal propensity to consume?
-
A knowledge of molar absorptivities is particularly important in biochemistry, where UV spectroscopy can provide an extremely sensitive method of analysis. For example, imagine that you wanted to...
-
Which of the following compounds would you expect to show ultraviolet absorptions in the 200 to 400nmrange? (a) (b) (c) CN CH3 (f) (d) (e) " N. Indole Aspirin
-
Show the structures of all possible adducts of the following diene with 1 equivalent ofHC1:
-
Q1) The equity of Washington Ltd at 1 July 2020 consisted of: Share capital 500 000 A ordinary shares fully paid $1 500 000 400 000 B ordinary shares issued for $2 and paid to $1.50 600 000 General...
-
out The following information relates to Questions 1 to 2. The management accountant of a furniture manufacturer is developing a standard for the labour cost of one massage chair. When operating at...
-
Exercise 10-8 Utilization of a constrained Resource [LO10-5, L010-6] Barlow Company manufactures three products: A, B, and C. The selling price, variable costs, and contribution margin for one unit...

Study smarter with the SolutionInn App


