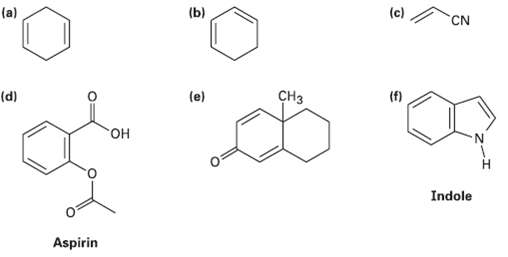
Which of the following compounds would you expect to show ultraviolet absorptions in the 200 to 400nmrange?
Question:
Which of the following compounds would you expect to show ultraviolet absorptions in the 200 to 400nmrange?
Transcribed Image Text:
(a) (b) (c) CN CH3 (f) (d) (e) "он N. Indole Aspirin
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (17 reviews)
All compounds having alternating single and mu...View the full answer

Answered By

Benish Ahmad
I'm a professional software engineer. I'm lectutrer at GCUF and I have 3 years of teaching experience. I'm looking forward to getting mostly computer science work including:
Programming fundamentals
Object oriented programming
Data structures
object oriented design and analysis
Database system
Computer networks
Discrete mathematics
Web application
I am expert in different computer languages such as C++, java, JavaScript, Sql, CSS, Python and C#. I'm also have excellent knowledge of essay writing and research. I have worked in other Freelancing website such as Fiverr and Upwork. Now I have finally decided to join the SolutionInn platform to continue with my explicit work of helping dear clients and students to achieve their academic dreams. I deliver plagiarism free work and exceptional projects on time. I am capable of working under high pressure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Related Video
For this experiment, we\'ve compared the freshness of flowers by keeping them in three separate bottles filled with water, aspiring water, and food plant
Students also viewed these Organic Chemistry questions
-
Which of the following compounds would you expect to be the most generally reactive, and why? DO
-
Which of the following compounds would you expect to be most acidic? Justify your choice.
-
Which of the following compounds would you expect to exhibit only London forces? a. Potassium chloride, KCl b. c. Silicon tetrafluoride, SiF4 d. Phosphorus pentachloride, PCl5 (
-
Cost information for Lake County Library is as follows. In addition to directly traceable costs, the library incurred $24,000 for a building lease. REQUIRED A. Allocate to departments any costs that...
-
What are some of the challenges with intercultural and cross-cultural communications and leadership?
-
Consider the ISBN-10 [0, 8, 3, 7, 0, 9, 9, 0, 2, 6]. (a) Show that this ISBN-10 cannot be correct. (b) Assuming that the error was a transposition error involving two adjacent entries, find the...
-
What are three ways of communicating interpersonally?
-
Natsam Corporation has $250 million of excess cash. The firm has no debt and 500 million shares outstanding with a current market price of $15 per share. Natsams board has decided to pay out this...
-
Alpha had a transaction that caused a 1,000 increase in both total assets and stockholders equity. The transaction could have been which of the following? The receipt of 1,000 from a customer before...
-
Amazon in OKC has just invested $250,000 in equipment having a negligible salvage value (SV = 0) regardless of when the equipment if replaced. O&M costs equal $50,000 the first year and increase...
-
A knowledge of molar absorptivities is particularly important in biochemistry, where UV spectroscopy can provide an extremely sensitive method of analysis. For example, imagine that you wanted to...
-
Show the structures of all possible adducts of the following diene with 1 equivalent ofHC1:
-
De Morgan's Law for the complement of the union of two sets \(A\) and \(B\) states that: \((A \cup B)^{\prime}=A^{\prime} \cap B^{\prime}\). Use a Venn diagram to prove that De Morgan's Law is true.
-
Write a brief statement that interprets the confidence interval. Choose the correct answer below. A. There is a 99% chance that the true value of the population mean weight of newborn girls will fall...
-
Transcribed image text: If estimated annual factory overhead is $1,072,500; overhead is applied using direct labor hours, estimated annual direct labor hours are 275,000 actum March factory overhead...
-
Your firm has limited capital to invest and is therefore interested in comparing projects based on the profitability index (PI), as well as other measures. What is the PI of the project with the...
-
The following rates are applicable to annual payroll in British Columbia Question 17 options: 1234 1.95% x total B.C. remuneration 1234 2.925% x (B.C. remuneration - $500,000) 1234 Tax Rate 1234...
-
Assume that different groups of couples use a particular method of gender selection and each couple gives birth to one baby. This method is designed to increase the likelihood that each baby will be...
-
The home page for the American Statistical Association is at http://www.amstat.org. Go to its website and identify and summarize the career options that one might have if interested in statistics.
-
Design and describe an application-level protocol to be used between an automatic teller machine and a bank's centralized computer. Your protocol should allow a user 's card and password to be...
-
What are the common units for expressing solution concentration?
-
Propose a mechanism that shows why p-chlorotoluene reacts with sodium hydroxide at 350 C to give a mixture of p-cresol and m-cresol.
-
Propose mechanisms and show the expected products of the following reactions. (a) 2, 4-dinitrochlorobenzene + sodium methoxide (NaOCH3) (b) 2, 4-dimethylchlorobenzene + sodium hydroxide, 350 oC (c)...
-
Nucleophilic aromatic substitution provides one of the common methods for making phenols. (Another method is discussed in Section 19-17.) Show how you would synthesize the following phenols, using...
-
Describe how the following affect the valuation of PPE. a) Cash Discounts b) Deferred Payment Contracts
-
Lou Barlow, a divisional manager for Sage Company, has an opportunity to manufacture and sell one of two new products for a five - year period. His annual pay raises are determined by his division s...
-
Consider a 5 year debt with a 15% coupon rate paid semi-annually, redeemable at Php1,000 par. The bond is selling at 90%. The flotation cost is Php50 per bind. The firm's tax bracket is 30%.

Study smarter with the SolutionInn App


