Which of the following compounds would you expect to be most acidic? Justify your choice.
Question:
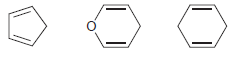
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
Deprotonatio...View the full answer

Answered By

Sandip Agarwal
I have an experience of over 4 years in tutoring. I have solved more than 2100 assignments and I am comfortable with all levels of writing and referencing.
4.70+
19+ Reviews
29+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following compounds would you expect to show ultraviolet absorptions in the 200 to 400nmrange? (a) (b) (c) CN CH3 (f) (d) (e) " N. Indole Aspirin
-
Which of the following compounds would you expect to have a ? ? ?* UV absorption in the 200 to 400 nm range? (a) (b) (c) (CH3)2C=C=Do CH2 A ketene Pyridine
-
Which of the following compounds would you expect to exhibit only London forces? a. Potassium chloride, KCl b. c. Silicon tetrafluoride, SiF4 d. Phosphorus pentachloride, PCl5 (
-
Use the trapezium rule, with interval-halving and extrapolation, to evaluate 0 log(cosh x) dx to 4dp
-
What is markup? How is it used to determine prices?
-
Find the natural logarithms of the given numbers. 76.1
-
2. When a complete system of encumbrance accounting is used, the authorizations remaining available for expenditures at any interim date will be equal to: a Appropriations less encumbrances b...
-
Gregorys Gems accumulated the following production and overhead cost data for the past 5 months. Required A. Use the high/low method to calculate the variable cost per unit and fixed costs for...
-
Please Answer it in Details 11. Case Study A manufacturing project would have a life of 15 years and it will lead to import substitution. Using the social conversion factors, calculate the economic...
-
WestJet Airlines Ltd. is one of Canada?s leading airlines, offering service to destinations in Canada, the United States, Mexico, the Caribbean, and Europe. The following is a partial extract from...
-
What are some things Amazon does to manage inventories in their warehouses?
-
How can performance measures sometimes cause the wrong things to happen?
-
Information was gathered on the starting median salary for students who attended four different types of colleges. Assume the samples are random and Normal. Test the hypothesis that the population...
-
2 4 . In the current year, Madison sold Section 1 2 4 5 property for $ 6 , 0 0 0 . The property cost $ 2 6 , 0 0 0 when it was purchased 5 years ago. The depreciation claimed on the property was $ 2...
-
Swifty Company purchased machinery on January 1, 2025, for $82,400. The machinery is estimated to have a salvage value of $8,240 after a useful life of 8 years. (a) Your answer is incorrect. Compute...
-
Currently, the unit selling price is $ 5 0 , the variable cost is $ 3 4 , and the total fixed costs are $ 1 0 8 , 0 0 0 . a . Compute the current break - even sales in units.
-
(1) The Mean Value Theorem states: Let f be continuous over the closed [a, b] and differentiable over the open interval (a, b). Then, there exists at least one point c E (a, b) such that: f(b) - f(a)...
-
Assume you are an Israeli investor; the symbol for the Israeli currency, the shekel, is ILS. You see that stock for Top Image has a bid price of ILS 17 and an ask price of ILS 19 in Israel, a bid...
-
Use the descriptions in Exercises 108109 to write an equation of a polynomial function with the given characteristics. Use a graphing utility to graph your function to see if you are correct. If not,...
-
Do public and private companies follow the same set of accounting rules? Explain.
-
(a) From what Grignard reagent can 3-methl-l pentanol be prepared by reaction with ethylene oxide, then aqueous acid? (b) Give the structure of another epoxide and another higher-order curpate that...
-
Explain why all attempts to isolate trimethyloxonium iodide lead instead to methl iodide and dimethl ether.
-
Explain why all attempts to isolate trimethyloxonium iodide lead instead to methl iodide and dimethl ether.
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
Alternative Food Networks Knowledge Practice And Politics 1st Edition - ISBN: 0415747694 - Free Book

Study smarter with the SolutionInn App


