Which of the following compounds would you expect to have a ? ? ?* UV absorption in
Question:
Which of the following compounds would you expect to have a ? ? ?* UV absorption in the 200 to 400 nm range?
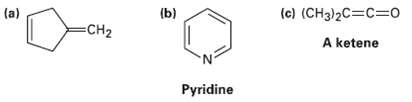
Transcribed Image Text:
(a) (b) (c) (CH3)2C=C=Do CH2 A ketene Pyridine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
Only compounds having alternatin...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which of the following compounds would you expect to have a dipole moment? If the molecule has a dipole moment, specify its direction. (b) H2O (c) CH4 (d) CH3Cl (e) CH2O (f) HCN
-
Which of the following compounds would you expect to be the most generally reactive, and why? DO
-
Which of the following compounds would you expect to be most acidic? Justify your choice.
-
If two krypton atoms are held together by a stabilization energy of 1.005 kJ/mol at what temperature would you expect these atoms to transition from bound to unbound? Show your thought process for...
-
What are the goals of management, unions, and society in this situation?
-
The process of adding rational functions (ratios of polynomials) by placing them over a common denominator is the analogue of adding rational numbers. The reverse process of taking a rational...
-
What is the difference between operational planning and strategic planning?
-
Compute trend percentages for Peak Valley Sales & Services total revenue and net income for the following five-year period, using year 0 as the base year. Round to the nearest full percent. Which...
-
please answer the question within the same format! I appreciate your help Required information The following information applies to the questions displayed below) Hemming Company reported the...
-
It is January 20, Year 13. Mr. Neely, a partner in your office, wants to see you, CPA, about Bruin Car Parts Inc. (BCP), a client req_uiring assistance. BCP prepares its financial statements in...
-
Would you expect allene, H2C = C = CH2, to show a UV absorption in the 200 to 400 urn range? Explain.
-
Predict the products of the following Diels?Alder reactions; (b) (a)
-
The semiconductor industry manufactures integrated circuits in large vacuum chambers where the pressure is 1.0 10 -10 mm of Hg. a. What fraction is this of atmospheric pressure? b. At T = 20C, how...
-
4) This question concerns the simulation of price trajectories in the Black-Scholes model. We therefore want to simulate price vectors: (St St, Str); where tiit, i=0,1,..., n. The total number of...
-
Consider a pure exchange economy with two goods, (x,y), and two consumers, (1,2). Con- sumers' endowments are e (4,2) and e = (6,6) and their preferences are represented by utility functions: u(x,y)...
-
O The national highways agency releases information on the pro- portion of people not wearing seatbelts, aggregated by city. The data comes from random traffic stops conducted between 8am and 9am on...
-
Ivanka's Budgeted Income Statement You are the accountant for Ivanka Ltd which operates a small mixed business. The following estimates relate to the base year (Year 1): Sales of product A $100 000...
-
1. Implement the function of a XNOR gate by a 2 to 4 decoder. Use logic gates if needed at the output. 2. The following question is to design an octal to binary encoder. a) Write down the truth table...
-
Understand the statistical method of partial least squares structural equation modeling (PLS-SEM)?
-
Write the general quadratic equation y2 - 8y - 4x + 28 = 0 in standard form. Determine the vertex, focus, and directrix of the parabola defined by this equation. Sketch a graph.
-
Which of these aqueous solutions has the highest boiling point? a) 1.25 M C 6 H 12 O 6 b) 1.25 M KNO 3 c) 1.25 M Ca(NO 3 ) 2 d) None of the above (they all have the same boiling point)
-
A graduate student tried to make o-fluorophenylmagnesium bromide by adding magnesium to an ether solution of o-fluorobromobenzene. After obtaining puzzling results with this reaction, she repeated...
-
A common illicit synthesis of methamphetamine involves an interesting variation of the Birch reduction. A solution of ephedrine in alcohol is added to liquid ammonia, followed by several pieces of...
-
Show how you would use a Suzuki reaction to synthesize the following biaryl compound. As starting materials you may use the two indicated compounds, plus any additional reagents you need. Make OCH3...
-
why should Undertake research to review reasons for previous profit or loss?
-
A pension fund's liabilities has a PV01 of $200 million. The plan has $100 billion of assets with a weighted average modified duration of 8. The highest duration bond that the plan can invest in has...
-
Metlock Limited has signed a lease agreement with Lantus Corp. to lease equipment with an expected lifespan of eight years, no estimated salvage value, and a cost to Lantus, the lessor of $170,000....

Study smarter with the SolutionInn App


