A tert-l3utyI esters [RCO2C (CH3)3] are converted into carboxylic acids (RCO2H) by reaction with trifluoroacetic acid, a
Question:
A tert-l3utyI esters [RCO2C (CH3)3] are converted into carboxylic acids (RCO2H) by reaction with trifluoroacetic acid, a reaction useful in protein synthesis (Section 26.7). Assign E, Z designation to the double bonds of both reactant and product in the following scheme, and explain why there is an apparent change of double-bonds tereochemistry:
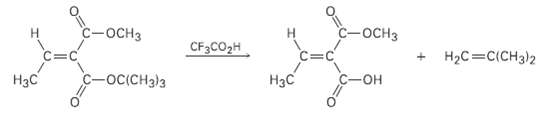
Transcribed Image Text:
с-оснз С-оснз НаС%3DCICH3)2 CF3CO2H Нас С-Он Нас -oc(CH3)3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
Treatment of the tertbutyl ester with trifluoroacetic acid cleaves the OCCH33 group and ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Wonder Textile has 10-year-old sewing machine with a cost price of $5,000. The rate of depreciation on the sewing machine is 10% per annum. The scrap value is $750. It has not been used in the...
-
Explain why firms exist. Explain why there is not just one huge firm.
-
Explain why there is a different money multiplier for each definition of the money supply.
-
A 110 g hockey puck sent sliding over ice is stopped in 15 m by the frictional force on it from the ice. (a) If its initial speed is 6.0m/s, what is the magnitude of the frictional force? (b) What is...
-
Suppose a college student graduates with a major for which he or she lacks enthusiasm. What might this person do about becoming a passionate leader?
-
Leeft Bank offers to reduce the collection time for your company's daily cash receipts by two days with its cash management system. This service will cost you $15,000 per year. Currently, short-term...
-
Through November, Cameron has received gross income of $120,000. For December, Cameron is considering whether to accept one more work engagement for the year. Engagement 1 will generate $7,000 of...
-
You work for a CPA firm that has been hired by Widget Tek Inc., a merchandising company that is getting ready to expand. The president of Widget Tek Inc. is concerned with obtaining a loan for the...
-
Bonita Industries incurs the following costs to produce 11100 units of a subcomponent: Direct materials $9324 Direct labor 12543 Variable overhead 13986 Fixed overhead 16200 An outside supplier has...
-
X Ltd. has 10 lakhs equity shares outstanding at the beginning of the accounting year 2016. The appropriate P/E ratio for the industry in which D Ltd. is 8.35. The earnings per share is Rs. 15 in the...
-
Which of the following E, Z designations are correct, and which are in correct? (a) CH (b) CH2CH=CH2 C=C CH2CH(CH3)2 o C=C (c) Br CH2NH2 C=c CH2NHCH3 (d) NC C C=C (CH3/2NCH2 CH-CH (0 2H c=c (e)...
-
Each of the following carbocations can rearrange to a more stable ion. Propose structures for the likely rearrangement products. H, (a) CH3CH2CH2CH2* (b) CH3CHCHCH3 CH CH CH2* (c)
-
Determine the missing property among P, T, s, x for the following states: a. Ammonia 25oC, v = 0.10 m3/kg b. Ammonia 1000 kPa, s = 5.2 kJ/kg K c. R-134a 5oC, s = 1.7 kJ/kg K d. R-134a 50oC, s = 1.9...
-
In 2022, Andrew, who is single, has a comfortable salary from his job as well as income from his investment portfolio. However, he is habitually late in filing his federal income tax return. He did...
-
1. What is the cost of direct materials used? 2. What is the cost of indirect materials used? 3. What is the cost of direct labour? 4. What is the cost of indirect labour? 5. What is the cost of...
-
Finding Critical Values. In Exercises 5-8, find the critical value za/2 that corresponds to the given confidence level. 5. 90% 6. 99%
-
You are an attorney at the law firm that represents Danfield's Auto Express. Your supervisor, Attorney Donna Defense, wants you to draft an internal memorandum of law to her assessing whether or not...
-
I desperately need help in this assignment, please help me!! Case Study Assignment You have recently been recruited by Velvet Chocolates Lid, a chocolate manufacturer, as an assistant management...
-
Customer Service Costs; Elimination of a Department. Durabond, Inc., believes in taking care of customer complaints quickly and efficiently, so it has created both a Customer Service facility and a...
-
What is removed during each of the three stages of wastewater treatment: primary, secondary, and tertiary? During which state would you expect items to be recovered that were accidentally flushed,...
-
How do the properties of water differ from those of most other substances?
-
What alkenes would give each of the following alcohols as the major (or only) product as a result of oxymercuration-reduction? CH-CH
-
Give the product(s) expected from the hydroboration--oxidation of each of the following alkenes. (a) Cyclohexene lel trans-4-methyl-2-pentene (b) Cis-3-hexene
-
Contrast the answers for Problem 5.8 with the answers for the corresponding parts of Problem 5.5. For which alkenes are the alcohol products the same? For which are they different? Explain why the...
-
The market price of a stock is $24.55 and it is expected to pay a dividend of $1.44 next year. The required rate of return is 11.23%. What is the expected growth rate of the dividend? Submit Answer...
-
Suppose Universal Forests current stock price is $59.00 and it is likely to pay a $0.57 dividend next year. Since analysts estimate Universal Forest will have a 13.8 percent growth rate, what is its...
-
ABC Company engaged in the following transaction in October 2 0 1 7 Oct 7 Sold Merchandise on credit to L Barrett $ 6 0 0 0 8 Purchased merchandise on credit from Bennett Company $ 1 2 , 0 0 0 . 9...

Study smarter with the SolutionInn App


