What alkenes would give each of the following alcohols as the major (or only) product as a
Question:
What alkenes would give each of the following alcohols as the major (or only) product as a result of oxymercuration-reduction?
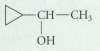
Transcribed Image Text:
CH-CH он
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (17 reviews)

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Identify the alkene that would give each of the following products upon ozonolysis followed by treatment with hydrogen peroxide: a. b. c. d. e. f. CH3CH2CH2COH CH3CCH CH3CCH2CH2CH2CH2CCH2CH3 OH HO +...
-
Each of the following alcohols has been prepared by reaction of a Grignard reagent with ethylene oxide. Select the appropriate Grignardreagent in each case.
-
For each of the following alcohols, give the systematic name and specif) whether the alcohol is primary, sec-ondary, or tertiary. a. b. c. Cl CH:CHCH2CH2 CH2CH2CH, CH3CCH2CH3 CH3 OH
-
A construction company is considering taking on a new project. The total project cost is $5 million, and there is a 70% chance that the project will be completed on time and within budget, resulting...
-
Currently 10,000 people take city buses each day and pay $2 dollars for a ticket. The number of people taking city buses at price p dollars per ticket is given x = 5000 6-p a. Evaluate the elasticity...
-
Define the term impairment loss.
-
E 5-6 Upstream and downstream sales 1. Pam Corporation owns 70 percent of Sun Companys common stock, acquired January 1, 2017. Patents from the investment are being amortized at a rate of $20,000 per...
-
In 2010, Nuts & Seeds Inc., purchased a new high-tech shelling machine from Soft-Core Corporation. Nuts and Seeds paid $1,000 in cash and gave Soft-Core a $29,000 note. The note is non-recourse and...
-
10. Use DerivaGem to complete this problem where you have an option on a non-dividend paying stock when the stock price is $30, the exercise price is $29, the risk-free interest rate is 5% per annum,...
-
Both Novador and Sashimia are members of the WTO. Sashimia is also one of 12 fishing nations that are members of the Southern Atlantic Tuna Fishing Organization (SATFO). SATFO was established in 1998...
-
Contrast the products expected when 3-methyl-1-butene undergoes (a) acid catalyzed hydration or (b) oxymercuration-reduction. Explain any differences.
-
Give the product(s) expected from the hydroboration--oxidation of each of the following alkenes. (a) Cyclohexene lel trans-4-methyl-2-pentene (b) Cis-3-hexene
-
You are in a meeting and one of your colleagues makes the following statement: Liquidity is excellent and our current ratio has increased from last year. Our current ratio is at 2.6, which is well...
-
Required: Prepare the supporting schedules for your portfolio for presentation to Mandla the supervisor and senior administrator. The schedules for the portfolio need to cover the following: Part A...
-
Write a program that will predict the size of a population of organisms. The program should ask for the starting number of organisms, their average daily population increase (as a percentage), and...
-
Management is keen to reduce inventory levels for materials as well and closing inventories are to be much lower. Expected levels are shown below: Material M1 Material M2 Material M3 2,200 kg 1,300...
-
How do you calculate incremental cost for the following: Complying with the Clean Air Act Amendments will be costly. There are three main options for complying with the Clean Air Act: analyze the...
-
How do I journalize this transaction? Mountain Swirl Ice Cream purchased and took delivery of one ice cream machine for $7,500. Record the sale and the cost of the sale. Markup is 150% of cost....
-
What are three solution techniques for solving lump-sum compounding problems? Which technique is the most efficient?
-
Dr. Chan obtained a $15,000 demand loan at prime plus 1.5% on September 13 from the Bank of Montreal to purchase a new dental X-ray machine. Fixed payments of $700 will be deducted from the dentists...
-
Identify all of the asymmetric carbon atoms (if any) in each of the following structures. (a) (b) CHCHCH- T CH3 CH3CHCH- T CH3 CH3 (c) HC-CH-CHOH T CHOCH3 (d) HC-CH-CHOH T NH -CH3
-
Arsenic (As) is below nitrogen and phosphorus in Group 5A of the periodic table. In an arsine (R 3 As;) the RAsR bond angles are about 92. How would you expect the inversion rate of arsines to...
-
Which of the following compounds could in principle be resolved into enantiomers at very low temperatures? Explain. (a) Propane (b) 2,3-dimethylbutane (c) 2,2,3,3-tetramethylbutane
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Total Dirt Bikes Mountain Bikes...
-
?? A local college is deciding whether to conduct a campus beautification initiative that would imvolve various projects, such as planting trees and remodeling bulidings, to make the campus more...
-
A company has net income of $196,000, a profit margin of 9.7 percent, and an accounts receivable balance of $135,370. Assuming 70 percent of sales are on credit, what is the companys days sales in...

Study smarter with the SolutionInn App


