Identify all of the asymmetric carbon atoms (if any) in each of the following structures. (a) (b)
Question:
Identify all of the asymmetric carbon atoms (if any) in each of the following structures.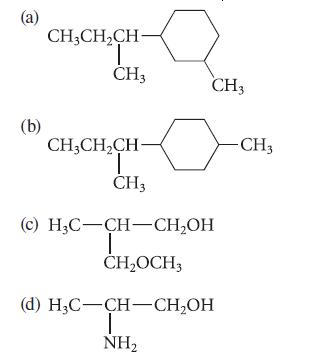
Transcribed Image Text:
(a) (b) CH₂CH₂CH- T CH3 CH3CH₂CH- T CH3 CH3 (c) H₂C-CH-CH₂OH T CH₂OCH3 (d) H₂C-CH-CH₂OH T NH₂ -CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The asymmetric carbons are indicated ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify all of the asymmetric carbon atoms in each of the following structures. (a) (b) (c) , , H C CH CH2OH NH2 CH3
-
The following four structures are naturally occurring optically active compounds. Star the asymmetric carbon atoms in these structures. CHO H CH, COOH OH OH H,N H serine erythrose menthol camphor
-
Indicate the asymmetric carbon atoms in the following compounds: (a) (b) CH3 CH3 CH2-CH-CH-C NH2 NH2 Br Br
-
The best measure of a firm's sustainable income is a .income before extraordinary items. b .net income. c. income before extraordinary item and change in accounting principle. d. income from...
-
Did the company cause the harm?
-
The CAPM computes expected rates of return using the following model (described in the chapter): the role of each of the three components of this model. ElR
-
Using the data on the two part numbers given, provide a comprehensive evaluation of the ordering policies. Compare the present annual average cost with the cost of using a system such as EOQ, and...
-
During 2010, Raines Umbrella Corp. had sales of $850,000. Cost of goods sold, administrative and selling expenses, and depreciation expenses were $610,000, $110,000, and $140,000, respectively. In...
-
Assume that Ernest Corporation has a required hurdle rate of 14% for all new investments. What does this hurdle rate mean? A. If a potential investment has an IRR of 14% or higher, it will be...
-
As described in the previous account, Pasteur discovered two stereoisomers of tartaric acid. Draw their structures [you cannot tell which is (1) and which is (2)]. Which stereoisomer of tartaric acid...
-
Arsenic (As) is below nitrogen and phosphorus in Group 5A of the periodic table. In an arsine (R 3 As;) the RAsR bond angles are about 92. How would you expect the inversion rate of arsines to...
-
Calculate H for the process in which Cl 2 (g) initially at 298.15 K at 1 bar is heated to 690.K at 1 bar. Use the temperature-dependent heat capacities in the data tables. How large is the relative...
-
The pipe assembly is mounted vertically as shown in (Figure 1). Part A Figure 200 mm 80 mm- 1.2 m Determine the pressure at A if the velocity of the water ejected from B is 0.77 m/s. Express your...
-
Suppose you were asked to estimate the probability that your coin came up "Heads" from your observations. Provide such an estimate including a 90% confidence interval. Explain your calculations.
-
Given the following C++ code snippet, what is the value of num3? Your answer must be exact. int num1, num2, num3; int *p_num1 - &num1; int *p_num2 &num2; *p_num1 15; *p_num2 10; * num3 = *p_num1...
-
# 1. On April 1, C.S. Lewis Company borrows (Notes payable) $70,000 from Lyon National Bank by signing a 3 month 8% note. Prepare the journal entries to record: a) the issuance of the note b) the...
-
Find and correct the program below, check your answer by running the script 1. 2. #include int main() 3. { 4. float Number1; 5. double Number2; 6. 7. 8. printf("Enter a number: "); scanf("%f",&num1)...
-
Name the bones of the cranium and the facial skeleton.
-
DC has unused FTC carryover from 2017 in the separate category for GC income as the result of income generated by a foreign branch. The income was foreign source general category income. In 2018 the...
-
P-Bromotoluene reacts with potassium amide to give a mixture of m- and p-methyl aniline. Explain.
-
Propose a mechanism to account for the reaction of benzene with 2, 2, 5, 5- tetra methyl tetra hydrofuran. H2SO4
-
Propose a mechanism to account for the following reaction: C CH2CI AICI3
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


