Aldehydes can be prepared by the Wittig reaction using (methoxymethylene)-triphenylphosphorane as the Wittig reagent and then hydrolyzing
Question:
Aldehydes can be prepared by the Wittig reaction using (methoxymethylene)-triphenylphosphorane as the Wittig reagent and then hydrolyzing the product with acid. For example,
(a) How would you prepare the necessary phosphorane?
(b) Propose a mechanism for the hydrolysisstep.
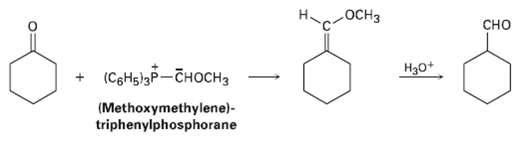
Transcribed Image Text:
OCH3 сно (СоНiзр— сноснз H30+ (Methoxymethylene)- triphenylphosphorane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
a b Ph3P BrCHOCH3 HOCH3 H0H protonation ...View the full answer

Answered By

Sinmon Warui Kamau
After moving up and down looking for a job, a friend introduced me to freelance writing. I started with content writing and later navigated to academic writing. I love writing because apart from making a living out of it, it is also a method of learning and helping others to learn.
5.00+
40+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you prepare 72.5 g of an aqueous solution that is 5.00% potassium iodide, KI, by mass?
-
How would you prepare 455 g of an aqueous solution that is 6.50% sodium sulfate, Na2SO4, by mass?
-
How would you prepare 2.00 L of (a) 0.10 M KOH from the solid? (b) 0.010 M Ba(OH)2 8H2O from the solid? (c) 0.150 M HCl from a reagent that has a density of 1.0579 g/mL and is 11.50% HCl (w/w)?
-
Every human being fears to fail. This is the same case with the leaders. Most of the leaders are afraid of some things, but failing is the most feared among all of them. The loss of direction among...
-
Various conflict management styles are depicted on PPT 9-7. For each of the participants in the scenario, describe which style(s) they used and cite examples to support your answer.
-
Two engineers are analyzing step-test data from a bioreactor. Engineer A says that the data indicate a second-order over damped process, with time constants of 2 and 6 min but no time delay. Engineer...
-
If you were to follow up the Slonaker and Wendt (2003) study on discrimination against African American males, what philosophical stance may underpin your research choice? LO6
-
Indicate which of the four perspectives in the balanced scorecard is most likely associated with the objectives that follow. 1. Ethics violations. 2. Credit rating. 3. Customer retention. 4....
-
united incorporated produces widgets for the manufacturing industry . among the costs incurred during the month, where the following: Sales commissions $6,000 Raw materials $21,000 Depreciation on...
-
Table 2.13 shows a data set containing information for 45 mutual funds that are part of the Morningstar Funds 500 for 2008. The data set includes the following five variables: Fund Type: The type of...
-
How would you use a Grignard reaction on an aldehyde or ketone to synthesize the following compounds? (a) 2-Pentanol (b) 1-Butanol (c) 1-Phenylcyclohexanol (d) Diphenyl methanol
-
When 4-hydroxyhutanal is treated with methanol in the presence of an acid catalyst, 2-mcthoxytctrahydrofuran is formed. Explain. -H C HCH2H2CH C
-
Solve the following inequalities: (a) |x - 7| < 3 (b) |x - 7| 3 (c) |x - 7| 1 (d) |x - 7| < 0.1
-
Follows is a list of outstanding invoices at 12/31/09. List is by customer. Company: Winter Invoice: 101 Date: Amount: 4/15 300.00 155 7/1 500.00 162 10/14 600.00 197 12/16 250.00 Bradley 126 6/25...
-
Question 3. The acceleration of a robot as it moves along a straight line in the horizontal x-axis is given by -kt a = e (2 cos wt +3 sin wt), k = 0, w % 0, where k and w are positive constants and...
-
(1 pt) To find the length of the curve defined by from the point (0,0) to the point (1,9), you'd have to compute where a b= and f(x)= y=5x+4x / f(x)dx
-
screen. In Exercises 21 through 32, find the instantaneous rates of change of the given functions at the indicated points. 21. f(x) = 2x + 3, c = 2 22.) f(x) = -3x+4, c = 3 23. f(x) = x - 1, c = 1...
-
Solve . f(x)= cos(x) 2+ sin(x)
-
Opportunities for Strategic Innovation Examination Question Synopsis This case study examination question and model answer is from the IIAUK & Ireland Advanced Diploma in Internal Auditing and...
-
Before the latest financial crisis and recession, when was the largest recession of the past 50 years, and what was the cumulative loss in output over the course of the slowdown?
-
The greatest bond length is found in (a) O 2 ; (b) N 2 ; (c) Br 2 ; (d) BrCl.
-
When acrolein (propenal) reacts with hydrazine, the product is a dihydropyrazole: Suggest a mechanism that explains this reaction. H + H2N-NH2 Acrolein Hydrazine A dihydropyrazole
-
(a) Propose step-by-step mechanisms for both transformations of the Robinson annulations sequence just shown. (b) Would you expect 2-methylcyclohexane-1, 3-dione to be more or less acidic than...
-
Outline reasonable mechanisms that account for the products of the following Mannich reactions: (a) (b) (c) NMe2 Me2N NMe2 + 2 Me2NH CH3 CH
-
Assume that an investment of $100,000 is expected to grow during the next year by 8% with SD 20%, and that the return is normally distributed. Whats the 5% VaR for the investment? A. $24,898 B....
-
Simpson Ltd is a small IT company, which has 2 million shares outstanding and a share price of $20 per share. The management of Simpson plans to increase debt and suggests it will generate $3 million...
-
The following are the information of Chun Equipment Company for Year 2 . ( Hint: Some of the items will not appear on either statement, and ending retained earnings must be calculated. ) Salaries...

Study smarter with the SolutionInn App


