When 4-hydroxyhutanal is treated with methanol in the presence of an acid catalyst, 2-mcthoxytctrahydrofuran is formed. Explain.
Question:
When 4-hydroxyhutanal is treated with methanol in the presence of an acid catalyst, 2-mcthoxytctrahydrofuran is formed. Explain.
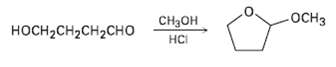
Transcribed Image Text:
-ОСHЗ Cнон носHCH2сH2CHо нCі
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
4Hydroxybutanal forms a cyclic hemiacetal when the hydroxyl ox...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
When benzene is treated with I 2 in the presence of C u Cl 2 , iodination of the ring is achieved with modest yields. It is believed that C u Cl 2 interacts with I 2 to generate I + , which is an...
-
On heating 1,2,4-butanetriol in the presence of an acid catalyst, a cyclic ether of molecular formula C4H8O2 was obtained in 81-88% yield. Suggest a reasonable structure for this product.
-
In a sulfuric acid plant, sulfur is burned in the presence of excess oxygen to produce sulfur dioxide, (SO,) which in turn is further reacted in the next step with oxygen in a converter to produce...
-
Analyze how you will use the challenge the process and enable others to act practices to improve the three leadership areas that you selected in module one. ? select one leadership theory or approach...
-
1. What sources of conflict were present in this scenario? 2. On PPT 9-4, The Conflict Process, overt behaviors are depicted as indicators of manifest conflict. What overt behaviors did you identify...
-
Consider the ratio control scheme shown in Fig. 15.6. Each flow rate is measured using an orifice plate and a differential pressure (D/P) transmitter. The pneumatic output signals from the D/P...
-
Why may it be argued that the concept of the manager is socially constructed rather than real? LO6
-
The controller of a school district had recorded the entire property tax levy, $20,000,000, as revenues when levied during the first month of the year. At year-end the auditor states that $3,000,000...
-
Essential College Supplies ( ECS ) is a store on the campus on a large Midwestern university. The store has both an apparel section ( t - shirts with the school logo ) and a convenience section. ECS...
-
Perform a hypothesis test to compare the accuracy of the two tests, and report a two-sided p-value? A new test is proposed that may be better or worse than the standard test. To assess their relative...
-
Aldehydes can be prepared by the Wittig reaction using (methoxymethylene)-triphenylphosphorane as the Wittig reagent and then hydrolyzing the product with acid. For example, (a) How would you prepare...
-
How might you carry out the following selective transformations? One of the two schemes requires a protection step. (Recall from Section 19.5 that aldehydes are more reactive than ketones toward...
-
Consider the following reaction: 2 CH 4 (g) C 2 H 2 (g) + 3 H 2 (g) A reaction mixture at 1700 C initially contains [CH 4 ] = 0.115 M. At equilibrium, the mixture contains [C 2 H 2 ] = 0.035 M. What...
-
Hackett Produce Supply is preparing its cash budget for April. The following information is available: Estimated credit sales for April Actual credit sales for March Estimated collections in April...
-
006 10.0 points A pendulum clock was moved from a location where g = 9.8168 m/s to another location where 9 9.806 m/s. During the move, g = the length of the clock's pendulum did not change;...
-
6-3x 2 Problem 6. (a) Find L So 6-3x-2y 3 2 dz dy dx. (b) Find the limits of integration. No need to find the integral. dx dz dy. Hint: The plane in the image is given by 3x + 2y + 3z = 6. 2.0 1.5...
-
Question Under what scale of measurement(s) can we say that : Jim weighs 4X as much as Edie? Sam is heavier than Sue? Jim and Sam don't weigh the same? Jim is much heavier than Sam, but Mary is only...
-
Lender Company provides postretirement health care benefits to employees who provide at least 10 years of service and reach the age of 65 while in service. On January 1 of the current calendar year,...
-
Internal Audit Capabilities and Needs Synopsis the responsibilities of internal audit functions have increased substantially in scope and complexity, creating the need for a commensurate increase...
-
(8%) Problem 6: A student attaches a f= 3.5 kHz oscillator to one end of a metal rail of length L = 25 m. The student turns on the oscillator and uses a piezoelectric gauge at the other end to...
-
The highest bond-dissociation energy is found in (a) O 2 ; (b) N 2 ; (c) Cl 2 ; (d) I 2 .
-
Write a structural formula for the product from each of the following reactions. (a) (b) (c) (d) (e) (f) NaOEt EtOH NaOEt EtOH NaOEt EtOH NaOEt EtOH (1)NaOEt, EtOH
-
Show all steps in the following syntheses. You may use any other needed reagents but you should begin with the compound given. (a) (b) (c) OEt OEt OEt OEt OEt
-
Provide the starting materials needed to synthesize each compound by acylation of an enolate. (a) (b) (c) CO2Et
-
Alta Ski Company's inventory records contained the following information regarding its latest ski model. The company uses a periodic inventory system. Beginning inventory, January 1, 2018 1,250 units...
-
Fibertech GmbH is a distributor of outdoors technical clothing. The company outsources the production of clothing to external manufacturers in Bangladesh and sells the clothing under its own brands....
-
PLEASE HELP WITH PART 2 & 3 Thanks Required information Exercise 1 0 - 7 ( Algo ) Part 2 Prepare journal entries to record the first two interest payments. Journal entry worksheet Record the interest...

Study smarter with the SolutionInn App


