How might you carry out the following selective transformations? One of the two schemes requires a protection
Question:
How might you carry out the following selective transformations? One of the two schemes requires a protection step. (Recall from Section 19.5 that aldehydes are more reactive than ketones toward nucleophilicaddition)
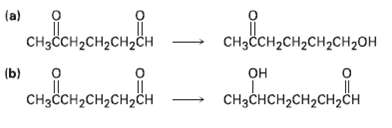
Transcribed Image Text:
"нено (a) одоненоненон CHзCсH-CH2CH2CH2ОH CHзссH2сH2CH2сн (b) "oneнономе онрнененемо CнзсCHн сH2сH2сн CнзCнсH-CH2CH,ҫH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
In general ketones are less reactive than aldehydes for both st...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you carry out the following transformations using an organo copper coupling reaction? More than one step is required in eachcase. (a) "CH (b) HH2CH2CHBr CH3CH2CH2CH2CH2CH2CH2CH3 (c)...
-
How might you carry out the following transformation? More than one step is needed. -CH2 CH2CH2OH
-
How would you carry out the following reactions to introduce deuterium into organicmolecules? (a) CHH2C3CCH2C Hs 2H5 (b) C2H5 CHH2C3CCH2CH3 C=C Hs D (c) CH3CH2CH2C=CH CH3CH2CH2C=CD CD CD2 C=CH (d)
-
1. Evaluate the term "health" and the historical perspective on health promotion. 2. Examine health promotion and illness prevention teaching based on teaching principles, varied teaching learning...
-
How is Netflix an Innovator? What are the consequences to competitors of Netflix's innovations?
-
The cascade reactor control configuration shown in Fig. 16.3 utilizes a measurement of the cooling water temperature. It has been suggested that the temperature of the reactor wall be measured to...
-
You have decided to undertake a project and have defined the main research question as What are the opinions of consumers to a 10% reduction in weight, with the price remaining the same, of Snackers...
-
The Minnesota adoption statute requires that any agency placing a child for adoption make a thorough investigation and not give a child to an applicant unless the placement is in the best interests...
-
Belle Company reports the following information for the current year. All beginning inventory amounts equaled $0 this year. Units produced this year 60,000 units Units sold this year 36,000 units...
-
In Problem simplify each fraction. 18x 3 y 3 /9x 3 z
-
When 4-hydroxyhutanal is treated with methanol in the presence of an acid catalyst, 2-mcthoxytctrahydrofuran is formed. Explain. -H C HCH2H2CH C
-
How would you synthesize the following substances from benzaldehyde and any other reagentsneeded? (c) (b) (a) CH- CH2
-
Estimate the specific heat difference c p c v for liquid water at 1000 psia and 300F.
-
What is the length of the partial wavelength for electromagnetic energy with a frequency of 15 MHz and a phase shift of 263 degrees (in meters)?
-
Let y = f(x) be a function that is differentiable at all real numbers. Suppose f has the form f(x) = {; [cx +6 4+2c for x 1 for x > 1. where b and c are some constants. What is the value of the...
-
Consider the sequence (n) defined by xn = (a) Show that 0In - n! nn (b) Use the result of part (a) and the Squeeze theorem to show that In 0 and n o.
-
Officials of Gwinnett County, one of the fastest growing counties in the country, are looking for ways to expand their sewer system. They are considering two alternative sewer designs. All annual...
-
On August 1, 20Y7, Rafael Masey established Planet Realty, which completed the following transactions during the month: Rafael Masey transferred cash from a personal bank account to an account to be...
-
The IIA Research Foundation Report March 2007 Synopsis Staying on the cutting edge of knowledge and understanding our profession is what The IIA Research Foundation is all about. So starts this 2007...
-
a. What is the cost of borrowing if Amarjit borrows $28 500 and repays it over a four-year period? b. How many shares of each stock would he get if he used the $28 500 and invested equally in all...
-
Which molecule is nonpolar? (a) SO 3 ; (b) CH 2 Cl 2 ; (c) NH 3 ; (d) FNO.
-
Write structural formulas for both of the possible products from the following Dieckmann condensation, and predict which one would likely predominate. EtOH, heat
-
When a Dieckmann condensation is attempted with diethyl succinate, the product obtained has the molecular formula C12H16O6. What is the structure of this compound?
-
Show how this diketone could be prepared by a condensation reaction:
-
The following information was available for the year ended December 31, 2022: Net sales $ 300,000 Cost of goods sold 210,000 Average accounts receivable for the year 15,000 Accounts receivable at...
-
Oslo Company prepared the following contribution format income statement based on a sales volume of 1,000 units (the relevant range of production is 500 units to 1,500 units): Sales $ 100,000...
-
Two jobs, A and B, have to be done on three machines, M1, M2, and M3 in the operation sequence given below. The operation times and the due dates are also given. Draw Gantt chart showing the...

Study smarter with the SolutionInn App


