How would you carry out the following reactions to introduce deuterium into organicmolecules? (a) CHH2C3CCH2C Hs 2H5
Question:
How would you carry out the following reactions to introduce deuterium into organicmolecules?
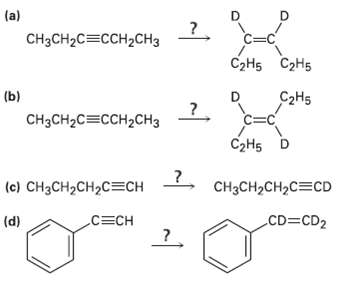
Transcribed Image Text:
(a) CHзсH2C3CCH2CНз СэHs С2H5 (b) C2H5 CHзсH2C3CCH2CH3 C=C СэHs D (c) CH3CH2CH2C=CH CH3CH2CH2C=CD CD CD2 C=CH (d)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 56% (16 reviews)
a b c d CH3CHCCCHCH3 CH3CHCCCHCH3 CH3CH...View the full answer

Answered By

Vikash Gupta
I am graduated in Physics in 2018, from KIRORIMAL COLLEGE, University of Delhi. Now I am persuing Master's degree in physics. I like to do physics problems. I have experience of 1 year in tutoring. I think Physics is the only subject where you understand things,how they are happening . In physics you learn Maths and apply it. So I would like to join your platform to solve many Physics problems.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you carry out the following transformations? More than one step may berequired. H-CH (a) (b) Br - (c) " - " - (d) CH3CH2CH2CH2C=CH CHH2CH2CH2CH2H20CH3 H (e) CH3CH2CH2CH2CHCH3...
-
How would you carry out the following transformation, a step used in the commercial synthesis of(S)-ibuprofen? CN
-
How would you carry out the following transformations? Co .CO2H (a) (b) Co CH2 C (c) CH2SH
-
YOU have just graduated from Deakin as an accounting and finance graduate. During your time at Deakin, you were organized and diligent with your studies and thus graduated with flying colors. This...
-
A manufacturer of nylon carpets produces rolls of carpeting at four factories and ships them to distributors in five locations The following table shows the capacities at the factories and the...
-
A stationary jet engine is shown. Air with a density of 0.0805 lb/ft 3 enters as shown. The inlet and outlet cross sectional areas are both 10.8 ft 2 . The mass of fuel consumed is 2% of the mass of...
-
Project Time Management a. Enter realistic durations for each task, and then link appropriate tasks. Make the additional tasks fit the time estimate: 20 days for analysis tasks, 30 days for design...
-
Your parents will retire in 18 years. They currently have $250,000, and they think they will need $1,000,000 at retirement. What annual interest rate must they earn to reach their goal, assuming they...
-
Flexible Budgeting At the beginning of the period, the Fabricating Department budgeted direct labor of $83,200 and equipment depreciation of $62,000 for 5,200 hours of production. The department...
-
The idealized cross-section of a two-cell thin-walled wing box is shown below with the data in Table 22.7. If the wing box supports a load of 44,500 N acting along web 25, calculate the shear flow...
-
How would you synthesize the following compounds from acetylene and any alkyl halides with four or fewer carbons? More than one step may herequired. (a) CH3CH2CH2C=CH (b) H2C%3CCH CH CH CHH2H3D (d)...
-
How would you prepare Cyclodecyne starting from acetylene and any alkyl halide needed?
-
Teardrop, Inc., wishes to expand its facilities. The company currently has 5 million shares outstanding and no debt. The stock sells for $31 per share, but the book value per share is $7. Net income...
-
Let f(x) = x+ 3, x20. The inverse of f is Of 1(x)=x - 3 (f (x) = -x-3 f-(x) = x - 3 Of 1(x) = 3 - x
-
Read the articles and please help me to write the whole assignment perfectly including the citations and references (APA Format). Pleaase choose the country and perspective of a particular industry....
-
A light, inextensible cord passes over a frictionless pulley as shown in figure below. One end of the rope is attached to a block, and a force P is applied to the other end. Block A weighs 600 lb and...
-
BASICOT POST DO NOT ASSIST DO NOT POST DO NOT ASSIST DO NOT POST DO NOT ASSIST For filming a physics demonstration about oscillation, an educational video crew attaches a large spring to a very small...
-
Day Mail Order Co. applied the high-low method of cost estimation to customer order data for the first 4 months of the year. What is the estimated variable order-filling cost component per order...
-
What can Jessica do to get this exhibition to use the convention center without inconveniencing either exhibition too much? LO.1
-
Record the following selected transactions for March in a two-column journal, identifying each entry by letter: (a) Received $10,000 from Shirley Knowles, owner. (b) Purchased equipment for $35,000,...
-
What is the molarity of Cl in each solution? a. 0.200 M NaCl b. 0.150 M SrCl 2 c. 0.100 M AlCl 3
-
The following compounds are listed in increasing order of acidity. In each case, the most acidic proton is shown in red. (a) Show the structure of the conjugate base of each acid, including any...
-
Amides such as acetamide are much weaker bases than amines, such as ethylamine (CH3CH2NH2). (a) Use resonance forms to show why the nonbonding electrons on the nitrogen atom of the amide are very...
-
Methyllithium (CH3Li) is often used as a base in organic reactions. (a) Predict the products of the following acid-base reaction. CH3CH2 - OH + CH3 - Li (b) What is the conjugate acid of CH3Li?...
-
If the auditor believes that the financial statements prepared on the basis of the entity's income tax are not adequately titled, the auditor should : A)Issue a resignation of opinion. B)Explain the...
-
initial stock offering to the public. This REIT specializes in the acquisition and management of warehouses. Your firm, Blue Street Advisors, is an investment management company that is considering...
-
Question 3 You have been hired to run a pension fund for Mackay Inc, a small manufacturing firm. The firm currently has Gh5 million in the fund and expects to have cash inflows of $2 million a year...

Study smarter with the SolutionInn App


