How would you synthesize the following compounds from acetylene and any alkyl halides with four or fewer
Question:
How would you synthesize the following compounds from acetylene and any alkyl halides with four or fewer carbons? More than one step may herequired.
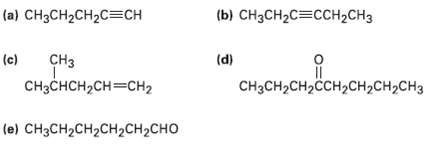
Transcribed Image Text:
(a) CH3CH2CH2C=CH (b) снзсH2C%3CCH CHз CHз CHснсH2сH3Dснг (d) (c) CнзCH-CH2CCH2CH2CH>CH3 (e) CH3CH2CH2CH2CH2CHO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
In all of these problems an acetylide ion or an anion o...View the full answer

Answered By

Issa Shikuku
I have vast experience of four years in academic and content writing with quality understanding of APA, MLA, Harvard and Chicago formats. I am a dedicated tutor willing to hep prepare outlines, drafts or find sources in every way possible. I strive to make sure my clients follow assignment instructions and meet the rubric criteria by undertaking extensive research to develop perfect drafts and outlines. I do this by ensuring that i am always punctual and deliver quality work.
5.00+
6+ Reviews
13+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you synthesize the following compounds from benzene? Assume that ortho and Para isomers can beseparated. (b) CH2CHCH3 (a) CH Br O2N SO3H
-
How would you synthesize the following compounds from Cyclohexanone? (a) 1-Methylcyclohexane (b) 2-Phenylcyclohexanone (c) cis-1, 2-Cyclohcxanediol (d) 1-Cyclohexylcyclohcxanol
-
How could you synthesize the following compounds from starting materials containing no more than four carbons? a. b. OH
-
Alpha corp reports the following results for the current year: net income per books (before federal income taxes ) 738,000 federal income tax expense per books (156,240) net income per books (after...
-
The Texas Electronics Company (TEC) is contemplating a research and development program encompassing eight major projects. The company is constrained from embarking on all projects by the number of...
-
A two-dimensional object is placed in a 4-ft-wide water tunnel as shown. The upstream velocity, v 1 is uniform across the cross section. For the downstream velocity profile as shown, find the value...
-
Project Communications Management a. Adjust the timescale on your Gantt chart to enable the chart to fit on one page. Then paste a copy of the Gantt chart in PowerPoint. You can use your Print Screen...
-
John Holland, accountant for Sunny Pie Foods, was injured in an auto accident. While he was recuperating, another inexperienced employee prepared the following income statement for the fiscal year...
-
You, as an Australian resident, hold shares in FIN222 Ltd which you purchased 2 years ago for $30 per share. They are now selling for $37 per share. You have a marginal tax rate of 37% while the...
-
While waiting for an appointment with your physician, you see a brochure advertising a new surgical procedure that implants a tiny microprocessor inside your skull just behind your left ear. The...
-
Synthesize the following compounds using 1-butyne as the only source of carbon, along with any inorganic reagents you need. More than one step may be needed. (a) 1, 1, 2, 2-Tetrachlorobutane (b) 1, 1...
-
How would you carry out the following reactions to introduce deuterium into organicmolecules? (a) CHH2C3CCH2C Hs 2H5 (b) C2H5 CHH2C3CCH2CH3 C=C Hs D (c) CH3CH2CH2C=CH CH3CH2CH2C=CD CD CD2 C=CH (d)
-
The owner of Patel Antique Clock Company of Churchill has asked you to prepare a worksheet from the following trial balance and additional data: PATEL ANTIQUE CLOCK COMPANY TRIAL BALANCE MAY 31, 2014...
-
7. This is a question about electromagnetic waves. (a) Starting from Maxwell's equations in a vacuum show that the electric field E and magnetic field B obey wave equations and identify the velocity...
-
Participate in workplace health and safety Third- party report Task 1: Case scenario: Workplace hazard collection and risk control form You are required to review this workplace inspection form and...
-
The red curve is the position-time x-t graph for the ladybug. Each tick mark on the time axis of the graph marks off 0.5 s. Note: you can hit reset graph and graph again to watch the graph form again...
-
Oma's Bakery is thinking about replacing the convection oven with a new, more energy-efficient model. Information related to the old and new ovens follows: (Click the icon to view the information...
-
One could argue that substantial travel for work is an undesirable characteristic of any job. What would the theory of compensating differentials predict about the relative wages of a sales position...
-
What is project management? LO.1
-
Repeat Exercise 16.6 using the t-test of the coefficient of correlation. Is this result identical to the one you produced in Exercise 16.6?
-
How many moles of KCl are contained in each solution? a. 0.556 L of a 2.3 M KCl solution b. 1.8 L of a 0.85 M KCl solution c. 114 mL of a 1.85 M KCl solution
-
The Ka of phenylacetic acid is 5.2 Ã 10-5, and the pKa of propionic acid is 4.87. (a) Calculate the pKa of phenylacetic acid and the Ka of propionic acid. (b) Which of these is the stronger...
-
Label the reactants in these acid-base reactions as Lewis acids (electrophiles) or Lewis bases (nucleophiles). Use curved arrows to show the movement of electron pairs in the reactions. (a) (b) (c)...
-
Predict the products of the following acid-base reactions. (a) (b) (c) (d) (e) (f) (g) (h) CH,COOH + (CH, ),N : H2O + NH3 HCOOH + CH,O- NH,CH,COOH 2 OH
-
Calculate Social Security taxes, Medicare taxes and FIT for Jordon Barrett. He earns a monthly salary of $11,100. He is single and claims 1 deduction. Before this payroll, Barretts cumulative...
-
Bass Accounting Services expects its accountants to work a total of 26,000 direct labor hours per year. The company's estimated total indirect costs are $ 260,000. The company uses direct labor hours...
-
The Balance Sheet has accounts where the accountant must make estimates. Some situations in which estimates affect amounts reported in the balance sheet include: Allowance for doubtful accounts....

Study smarter with the SolutionInn App


